For A-Level students or crazy people
In simple terms, an acid is a compound that has dissolved in water and has a pH of less than 7.
For A-Level…
You need to know that an acid is a compound that has dissolved in water and donated a hydrogen proton. This hydrogen proton combines with a water molecule, while the electron is released and able to roam freely. As a result, the acid will carry an electric charge.
A hydrogen atom consists of only a proton and an electron.

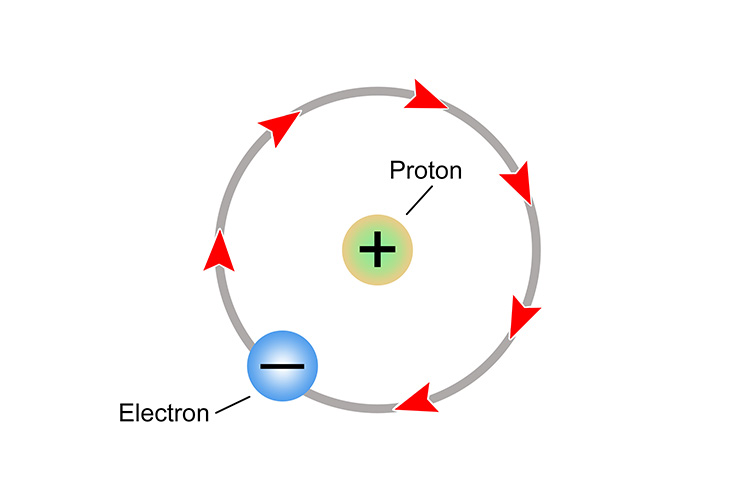
If the electron is released to roam around in a solution, only a hydrogen proton is left. This hydrogen proton is positively charged and called an ion or, more precisely, a cation.
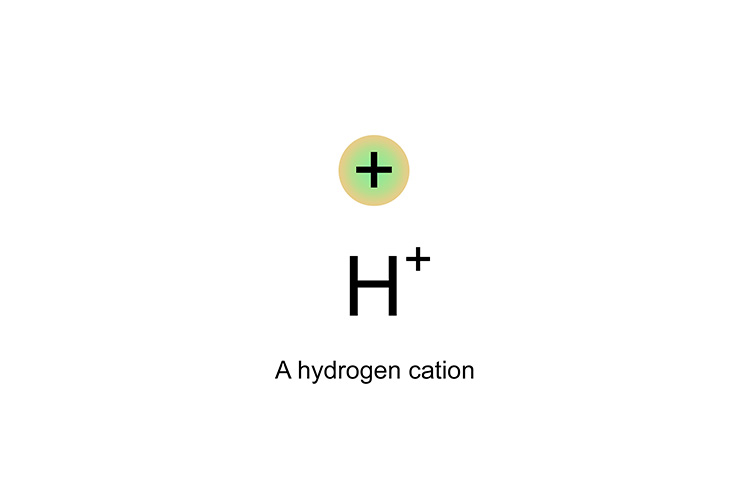
This hydrogen proton of H+ cation cannot exist on its own, so latches itself onto the water in the solution.
This is why at A-Level definitions proceed as follows:
- An acid is any substance that has donated a hydrogen proton to water
- A base is any substance that will accept a hydrogen proton from water
- An alkali is a base that has dissolved in water
- A salt is a compound that is created by the reaction between an acid and a base (or alkali).
Examples
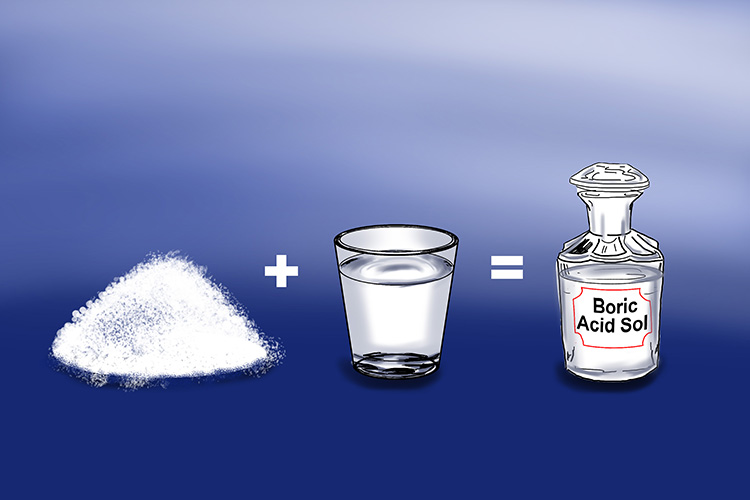
Hydrogen borate (Boric acid)
Some substances have the potential to be acids but, in their pure form, exist as solids. They do not become acids until they come into contact with water.
Hydrogen borate exists in the form of colourless crystals or a white powder block. If you dissolve hydrogen borate in water, it becomes boric acid (H3BO3).
Hydrogen chloride (hydrochloric acid)
Hydrogen chloride (HCl) does not release hydrogen ions until it meets water. Before it comes into contact with water, hydrogen chloride is a gas, not an acid.
At room temperature, hydrogen chloride is a colourless gas which forms hydrochloric acid upon contact with water (even moisture in the air).
When this happens, H2O and HCl combine to form hydronium cations (H3O+) and chloride anions (Cl-).
The resultant solution is a very strong acid: hydrochloric acid. This acid dissociation is large which means HCl dissociates or ionises almost completely in water.
Note: water is not a base even though it will accept hydrogen ions and gives them a temporary home.