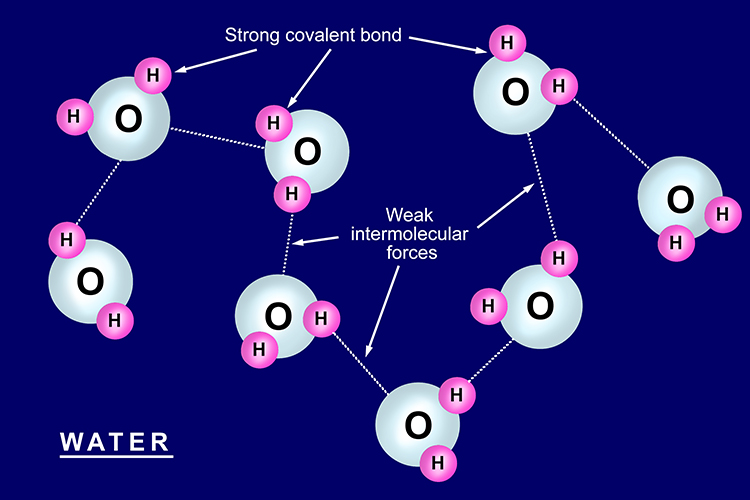
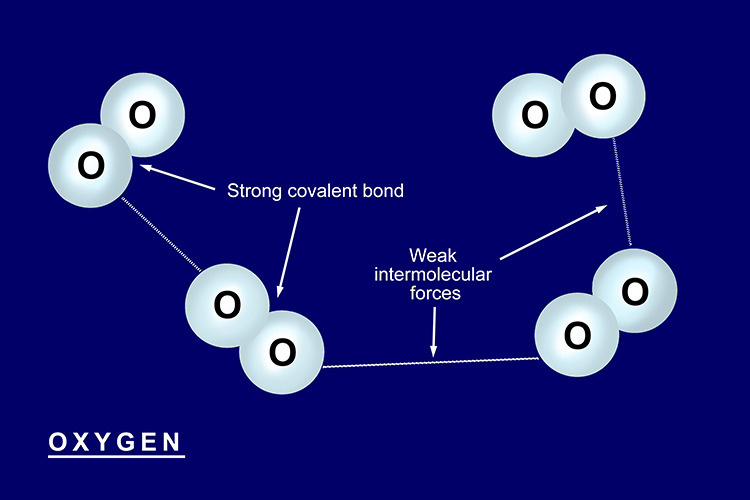
Properties of simple covalent structures
Substances with simple covalent structures have low melting points
Most covalent substances are a gas or liquid at room temperature. This is because although the covalent bonds between the atoms are very strong, the bonds between each molecule are very weak.

H2O has a low melting point; below 0°C, it is a solid and above 0°C it is a liquid or gas.
Examples of simple covalent structures: water and oxygen
Simple covalent structures do not conduct electricity
Simple covalent molecules cannot conduct electricity as they do not contain any free electrons (or ions).
Believe it or not, pure water (water without any impurities) does not conduct electricity well.
However, as soon as impurities are added, the water will conduct electricity. Don’t try this at home! Pure water is very expensive and if you were to get into a bath containing it, you would add impurities to the water and enable it to conduct electricity.