Redox reactions: reduction and oxidation
A redox reaction is a reaction that involves the transfer of electrons between elements.
The word ‘redox’ is made from the words that describe the two reactions, reduction and oxidation.
In an oxidation reaction a metal loses electrons and forms an ionic bond with oxygen that has stolen them.

Oxidation is the loss of electrons and reduction is the gain of electrons.
To remember this, think of OIL RIG.
This stands for:
Oxidation Is the Loss of electrons
Reduction Is the Gain of electrons
Redox reactions: an explanation
A redox reaction is a reaction in which reduction and oxidation take place.
There are two (yes, two) definitions of redox reactions.
Redox is the transfer of electrons. i.e. reduction and oxidation in terms of electron transfer (ionic)
Using OIL RIG
Oxidation is loss of electrons
Reduction is gain of electrons
Redox reactions also involve oxygen being gained or lost. Oxygen is gained or lost as atoms share (covalent) or transfer electrons (ionic).
Oxidation is gain of oxygen
Reduction is loss of oxygen
Note
If you see the words oxidation or reduction in an exam, the examiner is talking about a redox reaction and therefore a transfer of electrons or the transfer of oxygen.
It’s best to think of these two definitions as having nothing to do with each other; electrons are lost or gained through ionic bonding and when substances react with oxygen, either ionic bonding or covalent bonding can take place.
Definition 1: redox in terms of electrons.
Redox is the transfer (loss or gain) of electrons.

Definition 2: redox is the gain or loss of oxygen
Redox reactions are also reactions in which oxygen is lost and gained.
Oxidation is gain of oxygen
Reduction is loss of oxygen
Remember our picture for oxygen?

Here, the ox is gaining oxygen. It is undergoing oxidation. (Therefore, reduction is the opposite; the loss of oxygen)
If there is oxygen in the equation, you know that it is a redox reaction in terms of the loss or gain of oxygen, not the loss or gain of electrons.
Example 1
Magnesium + oxygen → Magnesium oxide
2Mg + O2 → 2MgO
If you are given this equation and asked which substance has reduced and which has oxidised, you don’t need worry about the electrons. You can just say:
Magnesium → Magnesium oxide
2Mg → 2MgO
Magnesium has gained oxygen and oxidation has taken place. The magnesium has been oxidised.
Example 2
Copper oxide + hydrogen → copper + water
CuO + H2 → Cu + H2O
If you are given this equation and asked which substance has reduced and which has oxidised, you don’t need to look at the electron arrangement, you can just say:
Copper oxide → Copper
CuO → Cu
Reduction has taken place as copper oxide has lost oxygen.
Copper oxide has been reduced to copper.
Example 3
Lead oxide + carbon → Carbon dioxide + lead
2PbO + C → CO2 + 2Pb
If you are given this equation and asked which substance has reduced and which has oxidised, because it includes oxygen, you can say:
Lead oxide → lead
2PbO → 2Pb
Lead (Pb) has lost oxygen and been reduced.
Carbon → Carbon dioxide
C → CO2
On the other hand, carbon has gained oxygen; it has been oxidised.
Lead oxide has been reduced, carbon has been oxidised.
Example 4
Iron oxide + carbon monoxide → iron + carbon dioxide
Fe2O3 + 3CO → 2Fe + 3CO2
Because an oxide is mentioned (and oxidation is the gain of oxygen and reduction is the loss of oxygen), if you are asked which substance has reduced and which has oxidised in this equation, you don’t need to worry about the electrons. You can just say:
Fe2O3 → 2Fe
Iron has lost oxygen. Reduction has taken place.
3CO → 3CO2
Carbon monoxide has oxidised to become carbon dioxide (it has gained oxygen).
Redox reactions in terms of electron transfer
Example 5
Magnesium + hydrochloric acid → magnesium chloride + hydrogen
Mg + 2HCl → MgCl2 + H2
If you are asked which substance has been oxidised and which has been reduced, because oxygen isn’t in the formula, we know that the reaction is ionic and we need to think about electrons.
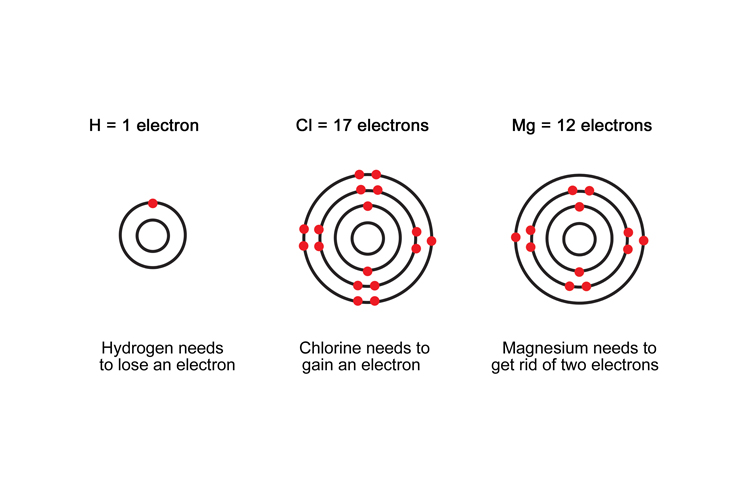
Magnesium
Magnesium → magnesium chloride
Mg → MgCl2
Magnesium loses two electrons and gives them to chlorine. It becomes positively charged.
Chlorine
Hydrochloric acid → magnesium chloride
2HCl → MgCl2
Before the reaction, chlorine had been given an electron by hydrogen; after the reaction, chlorine gains an electron from magnesium. It remains neutral.
Hydrogen
Hydrochloric acid → hydrogen
2HCl → H2
To begin with, hydrogen had given an electron to chlorine (it was negatively charged). Afterwards, it had gained an electron (from another hydrogen atom) to become neutral.
Magnesium has lost electrons. It has been oxidised.
Chlorine has kept the same number of electrons.
Hydrogen has gained electrons. It has been reduced.
Magnesium has been oxidised, hydrogen has been reduced.
Example 6
This is a really, really tough example, but here goes…
Potassium bromide + chlorine → Potassium chloride + bromine
2KBr + Cl2 → 2KCl + Br2
If you come across this equation and are asked which substance has been reduced and which has oxidised, because there is no oxygen in the equation, we know that the reaction is ionic and it is the number of electrons we need to look out for.
We know that:
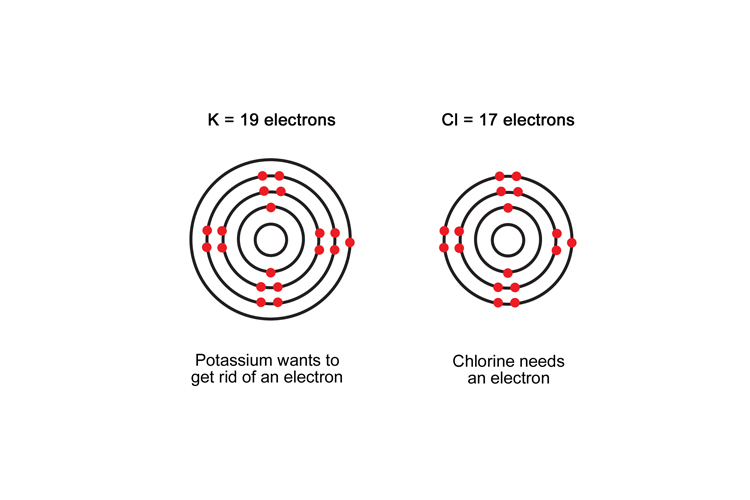
Potassium
2KBr → 2KCl
Before the reaction, Potassium had lost an electron to bromine; afterwards it loses an electron to and bonds with chlorine.
Chlorine
Cl2 → 2KCl
Chlorine gains an electron from potassium and becomes negatively charged.
Bromine
2KBr → Br2
Even though we don’t know the electron configuration of bromine, we can work out whether it has been reduced or oxidised by looking at the other elements in the reaction:
Potassium has kept the same number of electrons and has not been oxidised or reduced.
On the other hand, chlorine has gained an electron so has been reduced.
Because reduction cannot take place in a reaction without oxidation also taking place, we can deduce that bromine must have lost electrons and been oxidised.
Chlorine has been reduced, bromine has been oxidised.




