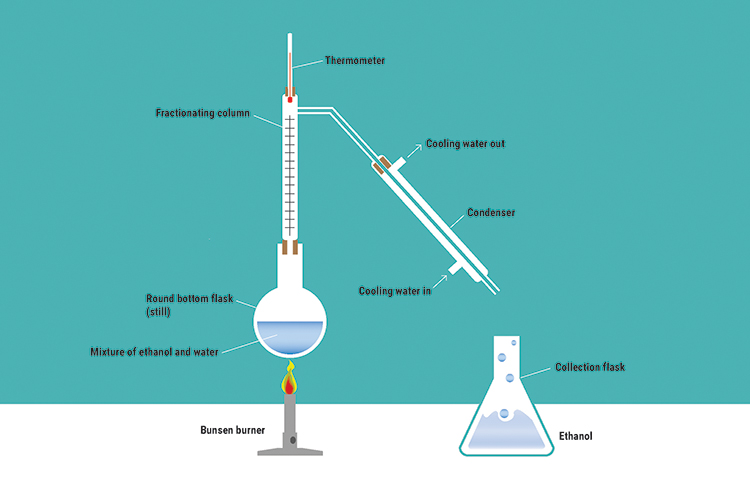
Fractional distillation part 1
Fractional distillation is the separation of a mixed solution by vaporising and condensing when the liquids have boiling points only a fraction apart (in distillation terms this is usually considered to be a difference of 40°C or less).
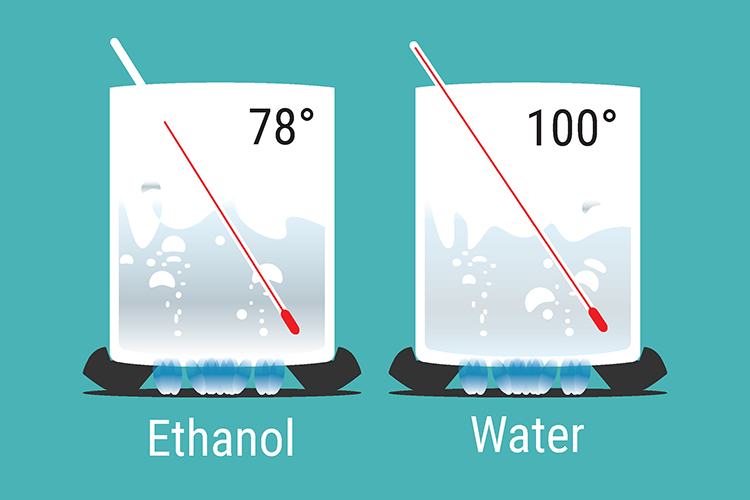
Example
As their boiling points are so close, both ethanol and water are boiled off. The liquid with the higher boiling point (water) condenses on the glass inside the column and runs back into the still, and the other condenses on the run out to the flask in the condenser. The fractionating column allows this to happen.