4) Moles of ideal gases = 24 litres
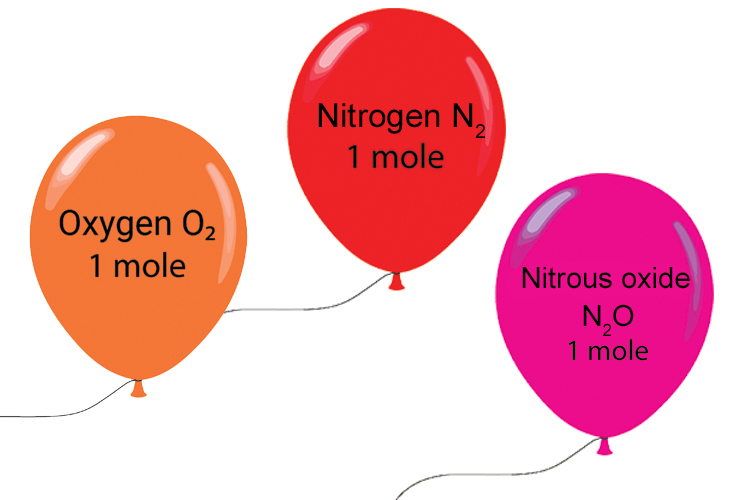
A mole of any “ideal” gas takes up 24 litres, about the same volume as a balloon.
(1 litre volume = 10 x 10 x 10 cm3)
Even though O2 and H2 look like this…
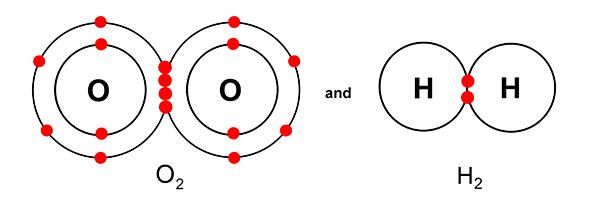
… and oxygen has more shells than hydrogen, both gases still take up the same volume.