Properties of the alkali metals
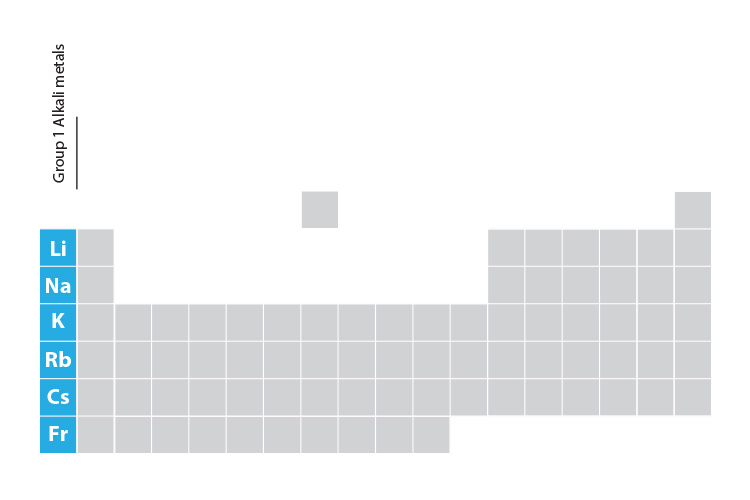
The elements in group 1 of the periodic table are referred to as the alkali metals. But what do you need to know about these elements and the way they behave? Alkali metals react violently with water to produce hydroxides, they react with oxides stealing their oxygen, they are soft and can be cut with a knife, they have low melting points and are less dense than water. How do you remember this? Read on!
There are three methods that you can choose to help you remember and understand the properties of the alkali metals.
Method 1. Understand and see the single electron in the outer shell.
Method 2. See and understand one of the most famous chemistry experiments.
Method 3. Learn a mnemonic story line.
Method 1
The reason why lithium (Li), sodium (Na) and potassium (K) react in a similar manner and so aggressively is because they all have only one electron in their outer shell. This means that they will not only react in a similar way but this single electron can very easily transfer to another atom.
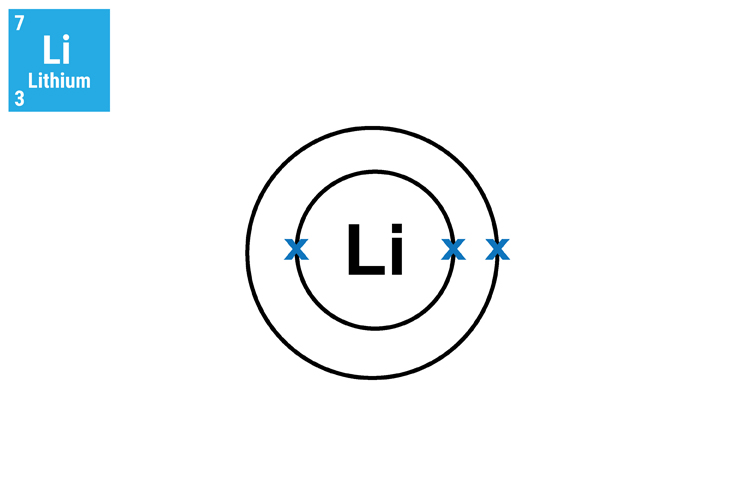
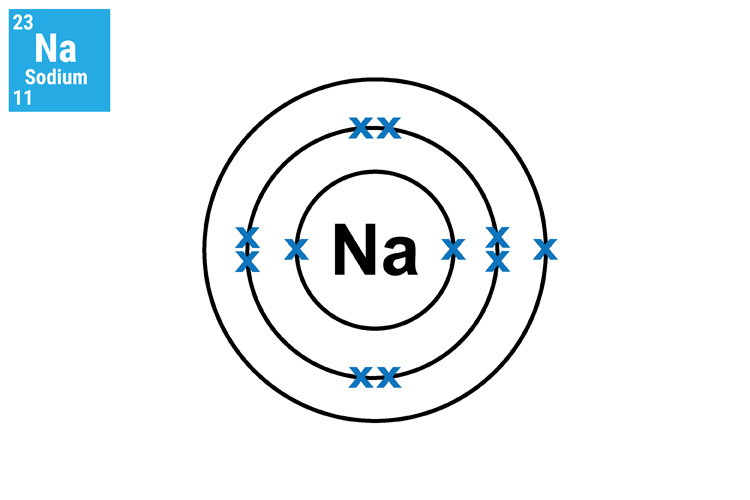
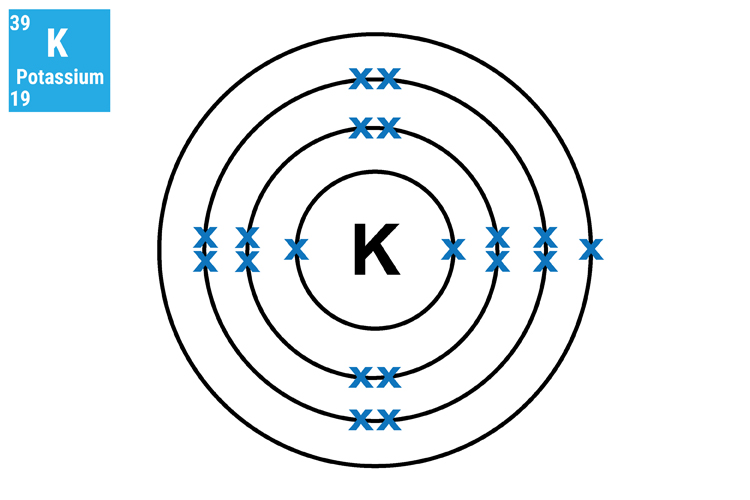
Method 2
If you take some lithium and drop it in water this famous experiment will teach you much about the properties of group 1 elements.
Lithium is very reactive
Group 1 metals are very reactive and one way that indicates that they are so reactive is they are stored in liquid keresone or mineral oil to prevent or limit their reaction with oxygen.
Alkali metals like to steal oxygen and form oxides:
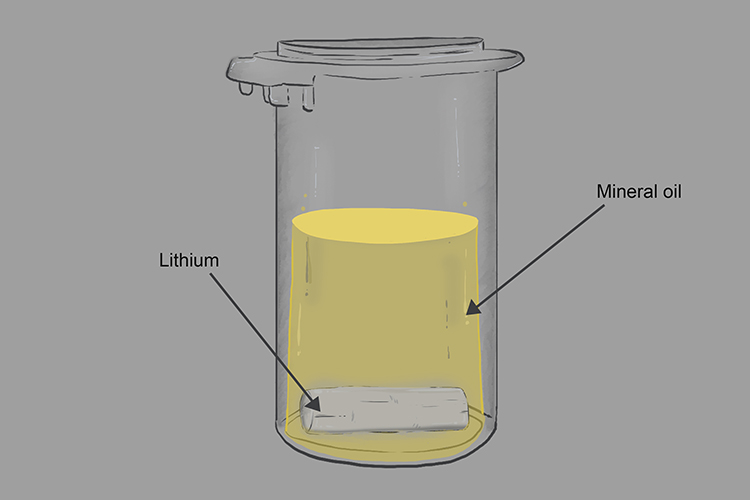
If we take the lithium out of the mineral oil you can see it is dull, grey and coated with a layer of the oxide.

Lithium is very soft:

Group 1 metals are relatively soft and can be cut with a knife.
Lithium is not dull but actually shiny:
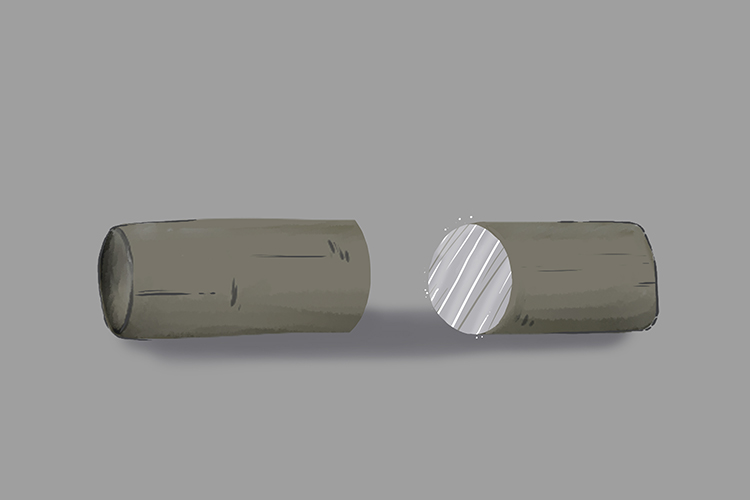
Lithium is a shiny metal, that is until it oxidises and turns dull grey.
Lithium is an excellent conductor of electricity:
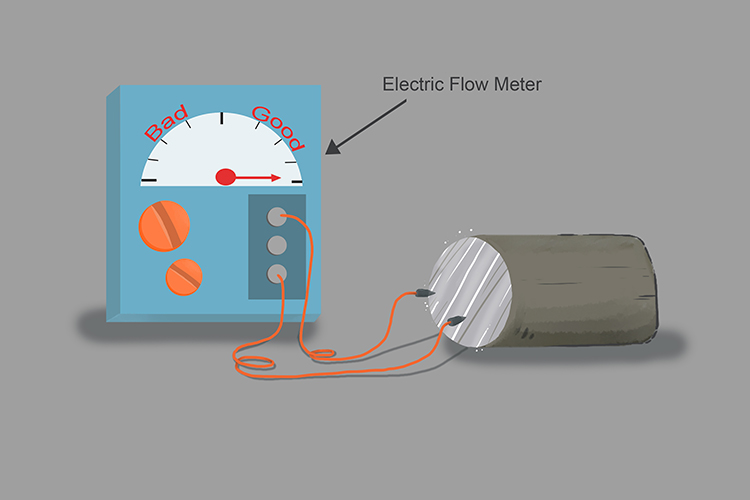
Lithium floats:
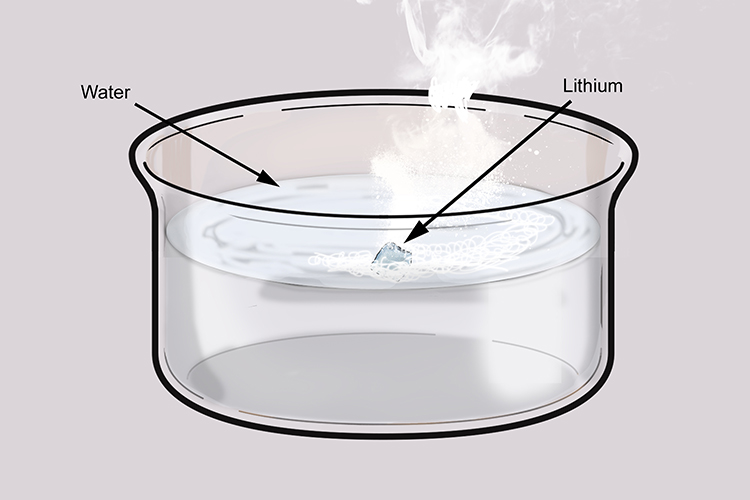
Add lithium to water and you will notice it will float. Group 1 metals are less dense than water.
Lithium reacts rapidly with water:

We can also observe that lithium reacts rapidly with water.
Lithium reacts with water to give off hydrogen:
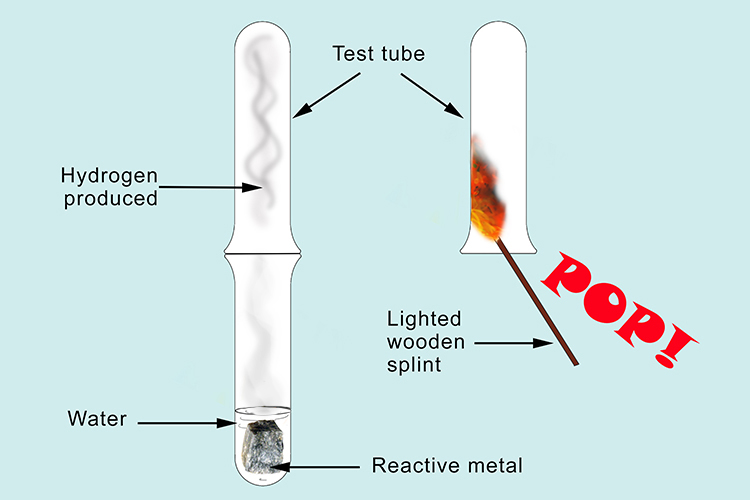
Lithium reacts with water producing lithium hydroxide and hydrogen. The lithium hydroxide is very water soluble and dissolves in water. The hydrogen gas escapes. If we trap some of the gas in a test tube and test the flammability by bringing a lighted wooden splint you will hear a pop proving that it is hydrogen.
Lithium hydroxide is a base:
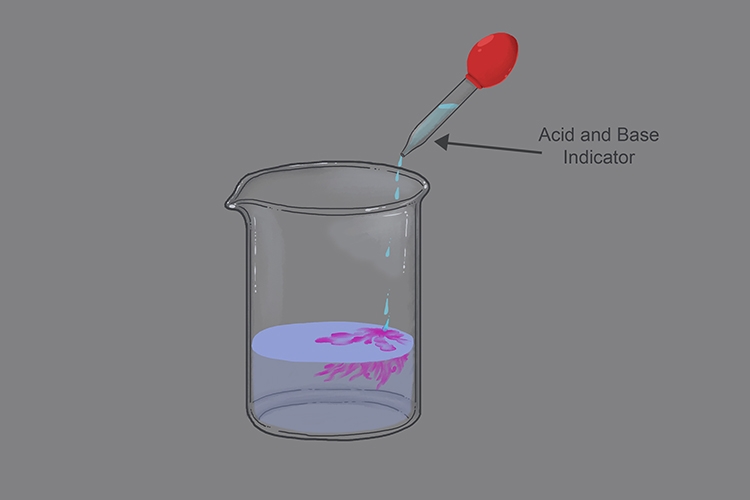
If you add an acid/base indicator in the beaker then the solution turns red/purple proving that lithium hydroxide is basic in solution.
Method 3
The third method involves giving you a mnemonic story that helps you to remember the properties of alkali metals.
Following a raid on the alley, where the alkies were lying down with their metal beer cans, the alkali metals (along with their electric robots*), were deemed dangerous and criminal.
They were put on the wanted list. The police issued the following statement, warning the public about alkali metals and their devious robots (electrons) and informing them of their properties.
*Each alkali metal has a robot as their sidekick (they have a single electron in their outer shell). It is these robots (electrons) that cause the metals to behave how they do. The robots (electrons) are a bad influence and are responsible for the alkali metals’ unlawful reputation.
.jpg)
NOTE:
Alkali Metals (Group 1 in the periodic table) are entirely different from alkalis (which may be more easily remembered as alkali solutions).
1. Alkali metals like to steal oxygen and form oxides
The police began by explaining that alkali metals had a long criminal history centred on the theft of oxygen.
For example:
Potassium + Oxygen → Potassium oxide
4K + O2 → 2K2O
Sodium + Oxygen → Sodium oxide
4Na + O2 → 2Na2O
Lithium + Oxygen → Lithium oxide
4Li + O2 → 2Li2O
2. Alkali metals react violently towards water
The public were warned not to approach an alkali metal and their robot, particularly in the presence of water, as they could be very violent and explosive.

3. Alkali metals are less dense than water so they float on its surface
The police said that, like many criminals, the alkali metals believed they were better than others as they were smarter and less dense than water.

4. Alkali metals are soft and easily cut with a knife
Not wanting to inflate the alkali metal’s ego too much, the police went on to say they were soft and easily cut with a knife much like butter.
5. Alkali metals react with water to form solutions called hydroxides
After reading the police statement, the alkali metals and their robots were worried, so their solution was to go and hide in a rock side (hydroxide).

For example:
Potassium + water → potassium hydroxide + hydrogen
2K + 2H2O → 2KOH + H2
Sodium + water → sodium hydroxide + hydrogen
2Na + 2H2O → 2NaOH + H2
Lithium + water → lithium hydroxide + hydrogen
2Li + 2H2O → 2LiOH + H2
6. Alkali metals have low melting points
However, this plan didn’t work as the alkali metals and their robot sidekicks soon began to melt under the heat from the police search.

To summarise, alkali metals:
- Have one electron in their outer shell
- Take oxygen atoms from other molecules to form oxides
- React violently with water
- Are less dense than water so they float
- Are soft so can easily be cut with a knife
- Form hydroxides when in a water solution
- Have low melting points compared with other metals




