Properties of the transition metals
The transition metals are the large group of elements in the middle of the periodic table between groups 2 and 3.
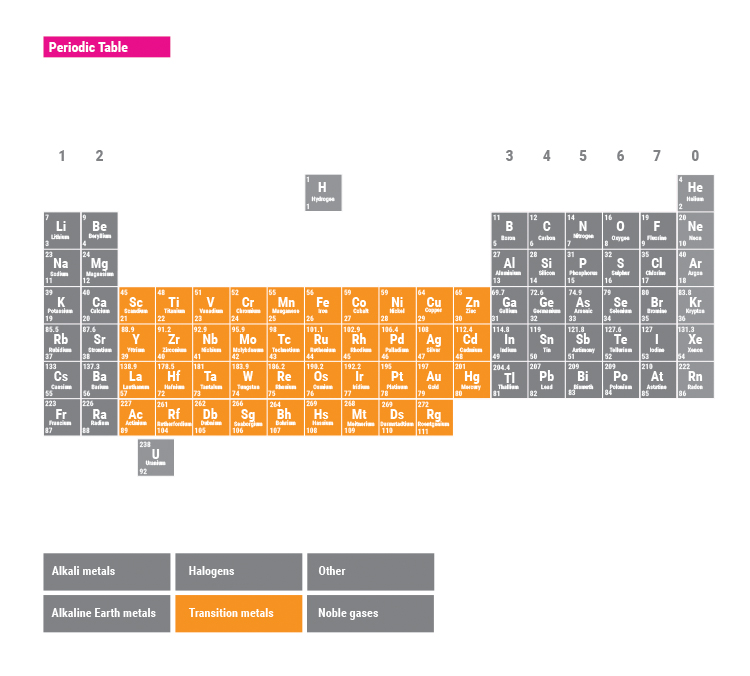
Common transition metals include zinc, copper, nickel and iron.
Transition metals all have the following properties:
- They are hard and dense
- They are less reactive than the group 1 & 2 metals
- They conduct heat and electricity well
- They have high melting points (with the notable exception of mercury)
- They are used as catalysts in industrial processes, such as the Haber process for making ammonia
- They form colourful ionic compounds
- Some can form more than one ion e.g. iron can form Fe2+ ions and Fe3+
Remembering the properties of the transition metals
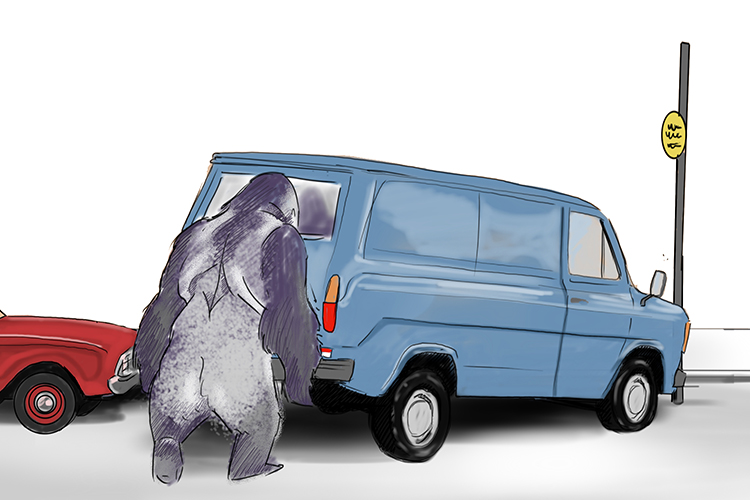
Because silver (Ag) is an example of a transition metal, the silverback gorilla is the focus of our story.
Once upon a time, a gorilla (silver Ag) tried to park his transit van (transition metals) but because there wasn’t much room, he had to lift it into the space. For a human, this would have been impossible. However, for the gorilla, because he was so hard and strong, it was no trouble at all.
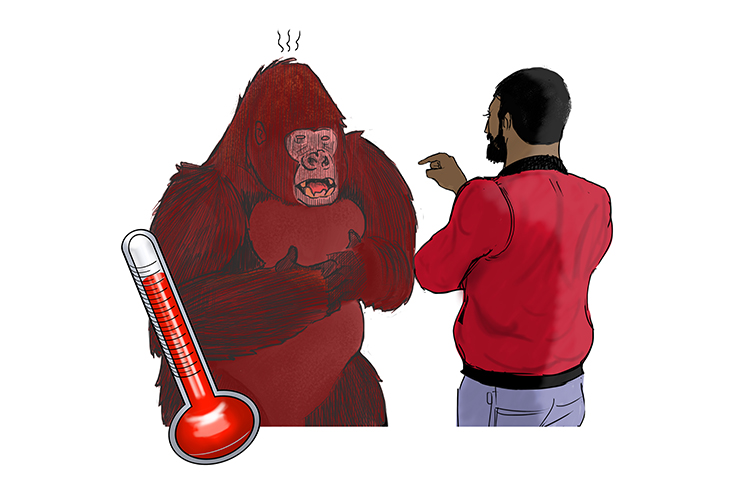
The man in the car next to him was not happy as the gorilla had blocked him in. “What do you think you’re doing?! Are you dense?!" he yelled.

Transition metals are dense.
It didn’t end there. He got out of his car and the conversation began to get heated.
The man was so livid that he threw a punch at the gorilla. The gorilla tried to defend himself, but his reactions were too slow and he ended up getting hurt.

Luckily, a benevolent cheetah happened to be running past. When she saw that the gorilla had been hurt, she was keen to assist (Cat-assist, catalyst).

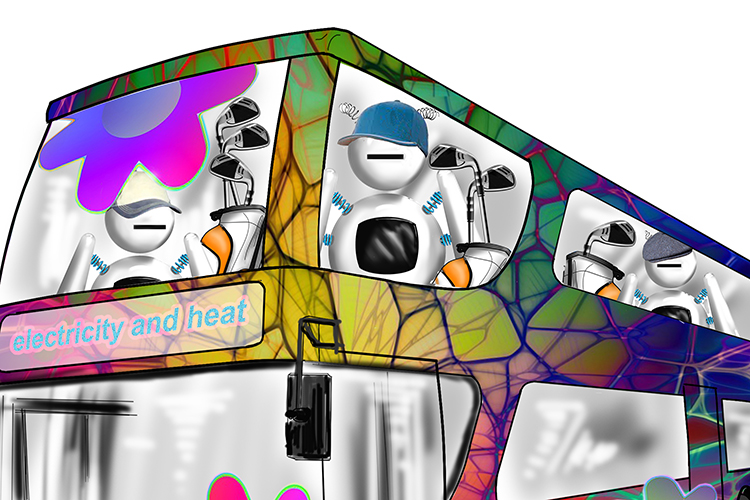
The pair’s bust-up was holding up the traffic. A bus stopped and its conductor got out to see what all the commotion was about.

The conductor’s bus was anything but ordinary; it was painted with bright colours.
On the top deck of the bus was a group of robots on their way to the local golf course. They each had golf bags containing one, two or three irons.