The reaction between zinc and steam
When zinc reacts with steam, zinc oxide and hydrogen gas are produced.
The reaction is similar to the one between magnesium and steam but is slower.
| Zinc | + | Steam | → | Zinc oxide | + | Hydrogen |
| Zn | + | H2O | → | ZnO | + | H2 |
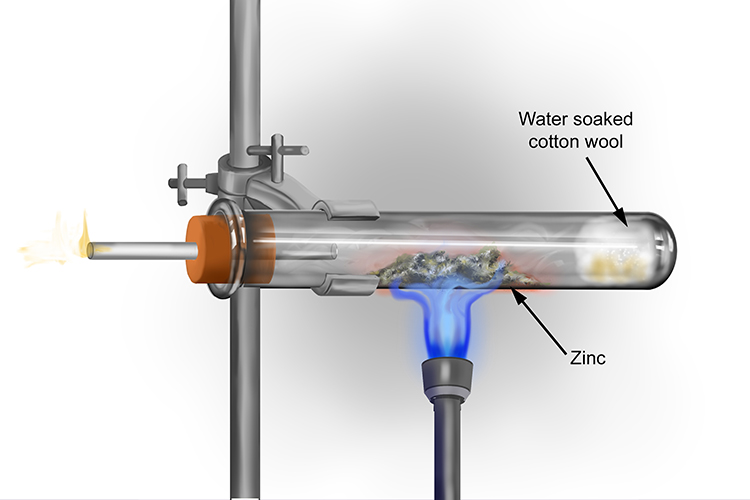
The zinc reacts slower with the steam than magnesium and does not glow brightly as it is converted to zinc oxide.
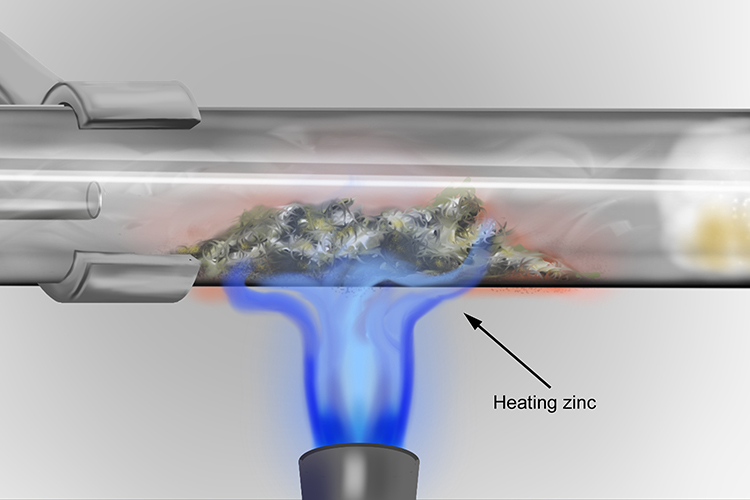
Heating the zinc will speed up the reaction but it will still not burn brightly like the magnesium.
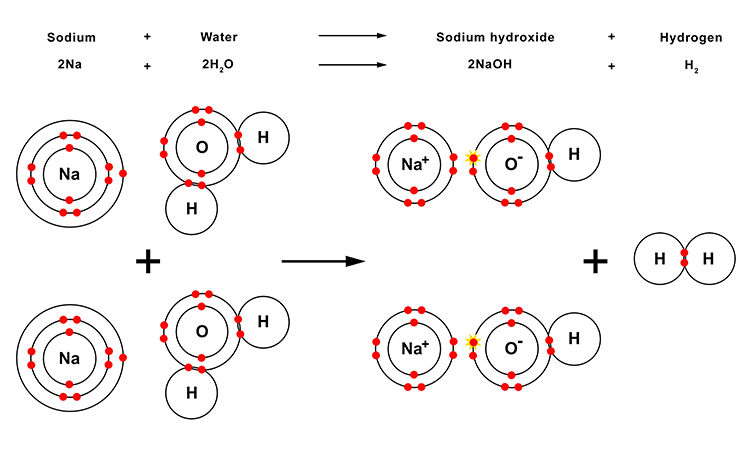
The reaction between zinc and steam in more detail
Note: You will not need to know this information but it may help to provide you with a better understanding of what takes place during this reaction.