The reaction between lead and oxygen
Lead can form many different oxides. The one most commonly formed when it reacts with oxygen is lead (II) oxide, also known as lead monoxide. However, it may also form tri-lead tetroxide, sometimes called red lead, or lead (IV) oxide, also known as lead dioxide.
| Lead | + | Oxygen | → | Lead (II) oxide |
| 2Pb | + | O2 | → | 2PbO |
| Lead | + | Oxygen | → | Lead (IV) oxide |
| Pb | + | O2 | → | PbO2 |
| Lead | + | Oxygen | → | Tri-Lead tetroxide |
| 3Pb | + | 2O2 | → | Pb3O4 |
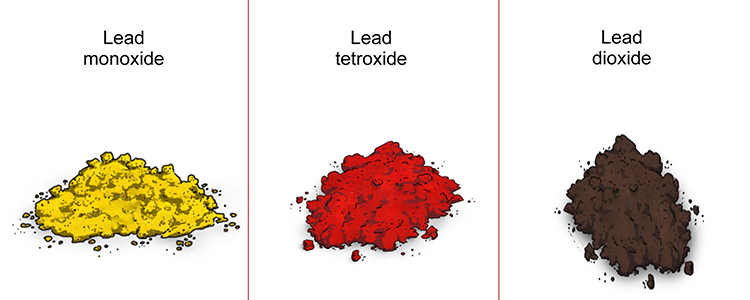
The lead oxides produced in this reaction appear as a red, yellow or dark brown powder, and are insoluble in water.




