The reactivity series: an introduction
The reactivity series of metals is a handy list of common metal elements arranged in order of their chemical reactivity, from the most to the least reactive.
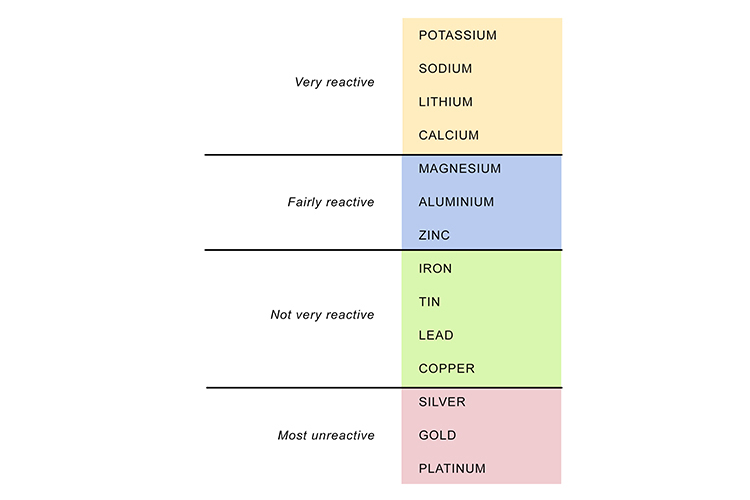
The different levels of reaction of metals in the reactivity series can be shown by what happens when they are placed in water:
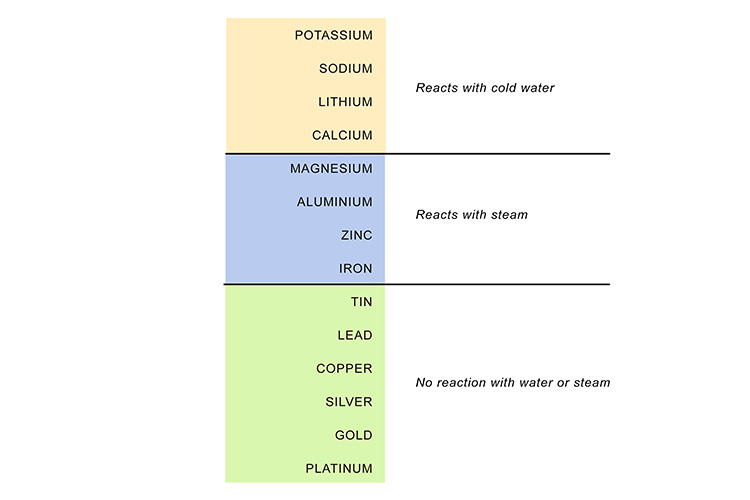
For example, sodium, near the top of the list, reacts violently with water...

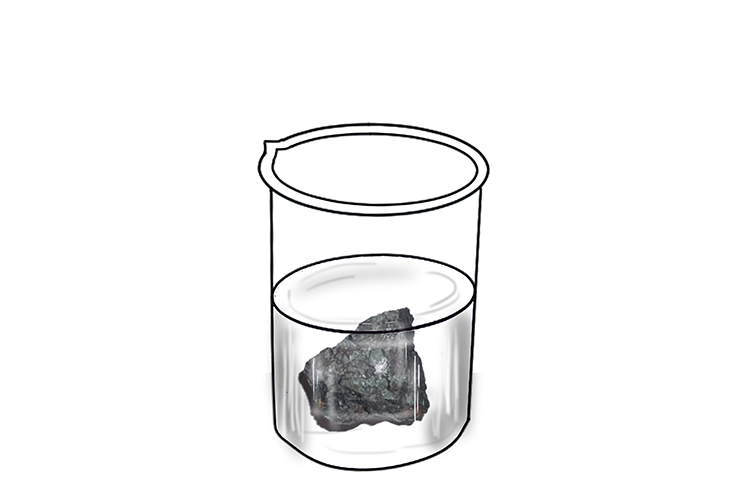
…while placing iron in water produces no discernible immediate reaction – although the iron will slowly rust if left in water long enough.
If a metal reacts with water, hydrogen is always released:
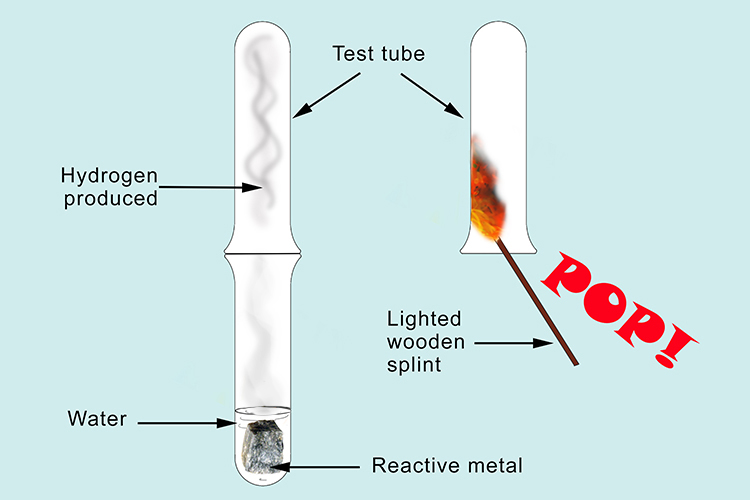
To summarise, some metals are very reactive – that is, they readily take part in chemical reactions – and these will be at or near the top of the reactivity series list. Other metals are very unreactive and will be at or near the bottom of the list. In between are metals that have medium to slow reactions.




