Electron shells and 2,8,8
.jpg)
Electronic shells explained
- Electrons always occupy these shells or orbits.
- The orbit or shell nearest to nucleus is filled first.
- Only a certain number of electrons are allowed in each shell:
- The first orbit or shell allows 2
- The second orbit or shell allows 8
- The third orbit or shell allows 8
- Atoms are happier when there is a full electron shell
- If the outer shell is not full it will want to react
Examples:
Sodium

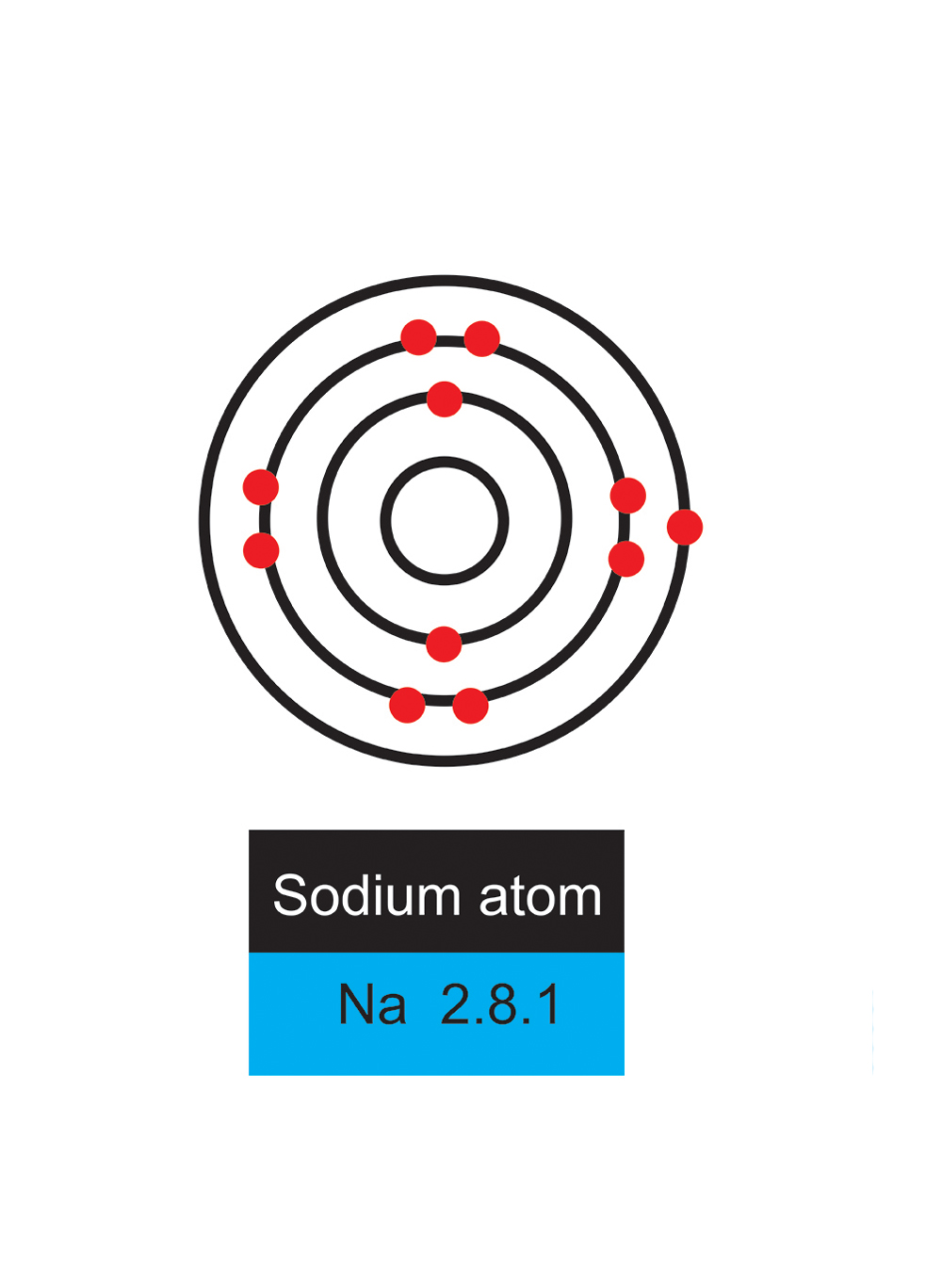
Sodium has 11 electrons
Sodium has only one electron in its outer shell and therefore wants to get rid of it.
Chlorine

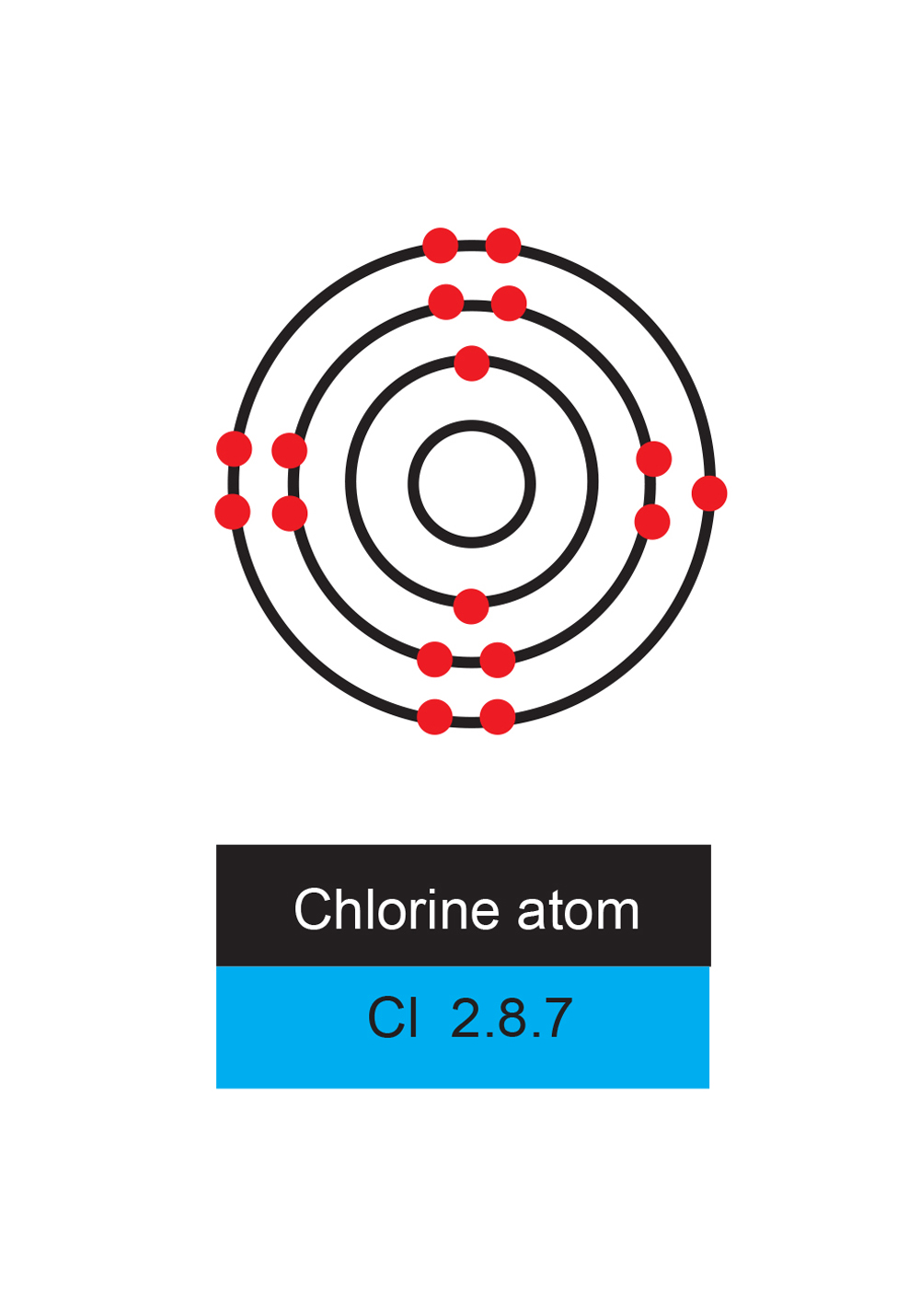
Chlorine has 17 electrons.
Chlorine needs one more electron to make a full shell and wants one badly.




