Hydrocarbons: an introduction
A hydrocarbon is a chemical compound containing only carbon and hydrogen atoms. The word ‘hydrocarbon’ tells you all you need to know:
Hydro(hydrogen)-carbon

One of the most common substances made up of hydrocarbons is a fossil fuel called crude oil. It is an extremely important raw material, used to make a number of different things, including fuels like kerosene and petrol, as well as plastics. Crude oil is broken up into hydrocarbons by fractional distillation and cracking.
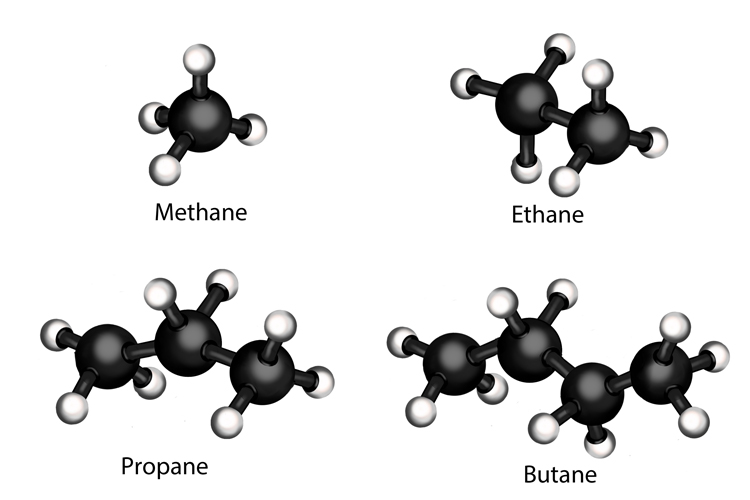
1st hydrocarbons in crude oil are alkanes. They have a single carbon bond joining their atoms together. Examples of alkanes found in crude oil are methane (natural gas), ethane, propane (sometimes known as liquefied petroleum gas or LPG) and butane.
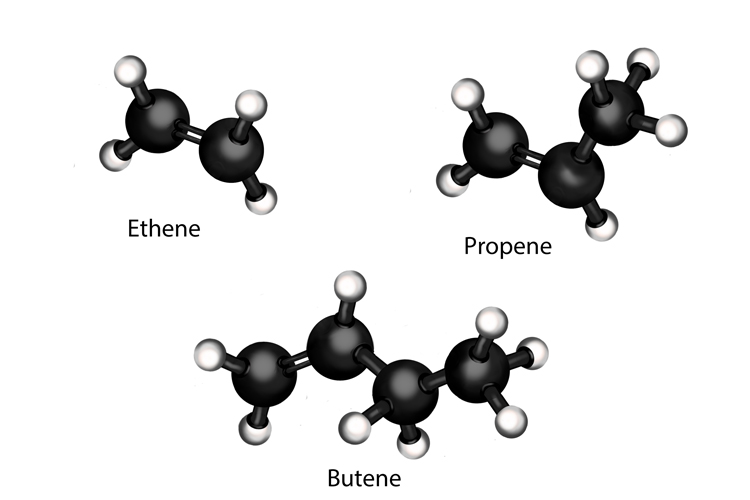
Alkenes are another type of hydrocarbon. Their carbon atoms are joined together by double bonds. Alkenes are usually formed when alkanes are broken down into smaller molecules during cracking. Examples of alkenes include ethene (used to produce polythene), propene and butene.