Copper atoms
To help us decide which way electricity flows, let's look at the structure of a copper atom which is used universally as an electrical carrier.
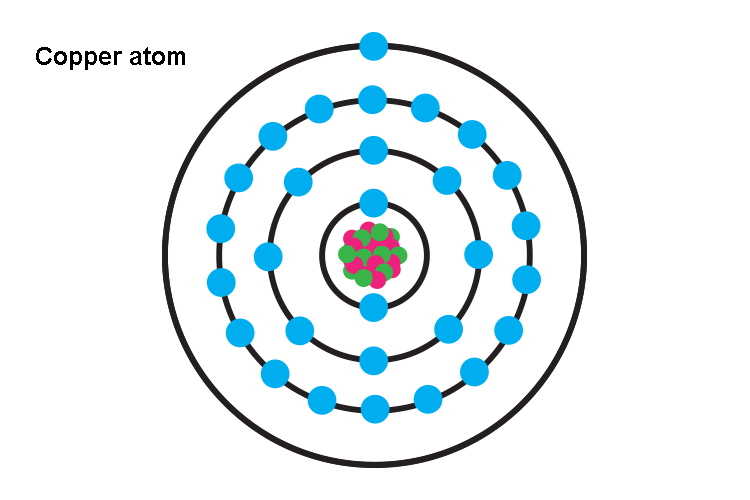
In this neutral state of charge, the atom has the same amount of +ve protons as -ve electrons, i.e. 29.
But the outside shell only has one electron and is vulnerable to being removed.
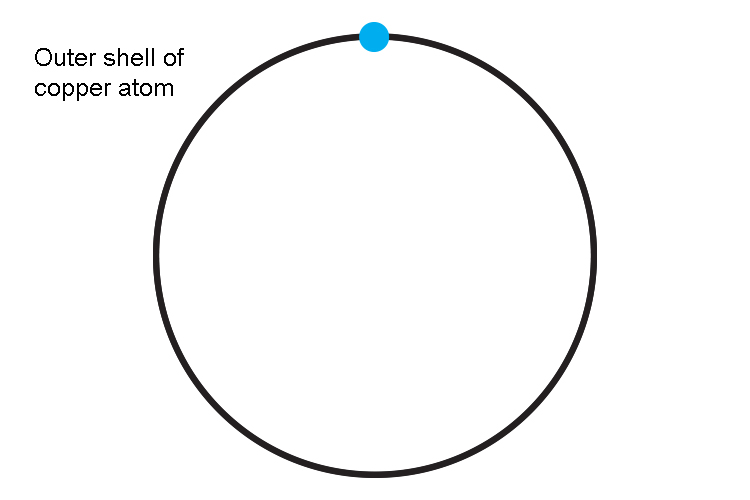
Electrons close to the nucleus are hard to remove while those in the outer ring require relatively little energy to be removed and become free electrons.




