What's happening inside a copper wire
The animated diagram below shows what happens when the two ends of a battery are connected with copper wire.
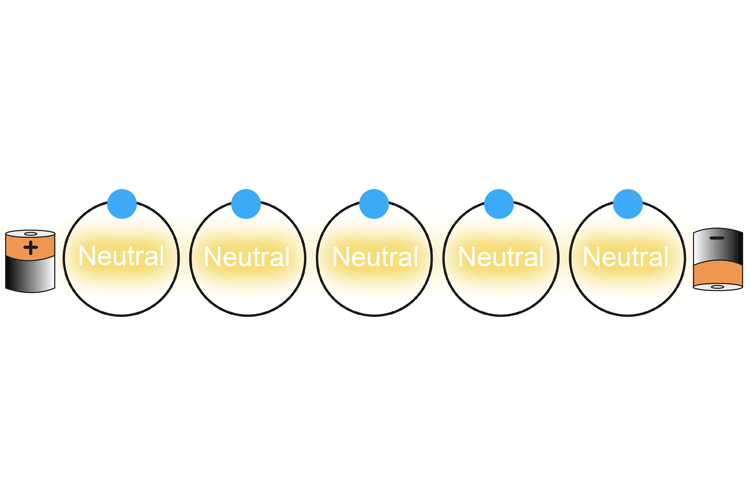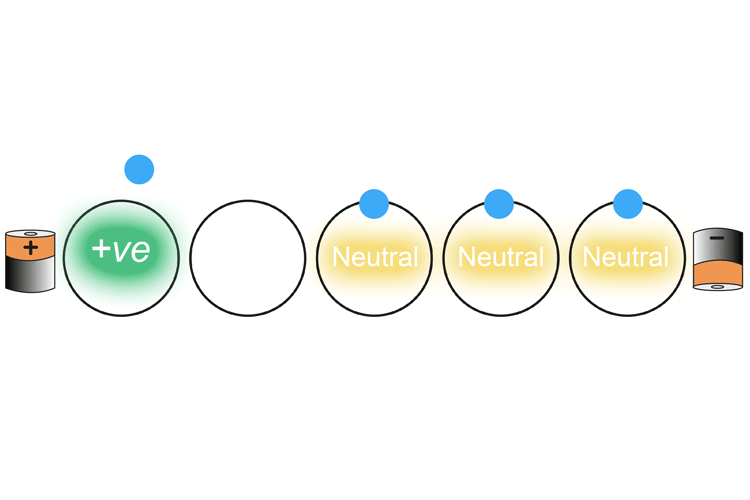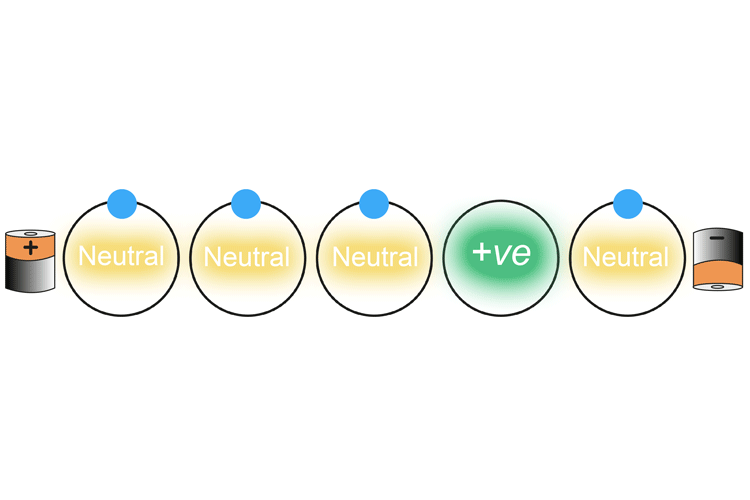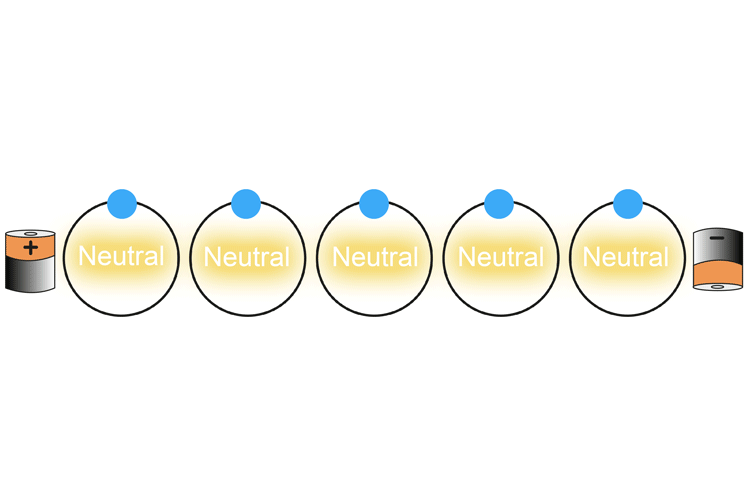
The negatively charged electron nearest the positively charged battery is drawn into the battery. This creates a positively charged atom (called an ion) which then in turn attracts the nearest negatively charged electron. As an electron leaves the atom and is replaced with another electron, each atom changes from a neutral charge to a positive charge and back again.
This continues on down the wire.




