Indicators and the pH scale
Indicators are substances that change colour when in acidic or alkaline conditions. The two most common indicators are litmus paper and universal indicator.
Litmus paper
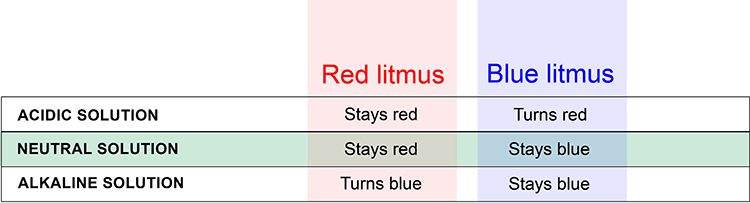
Litmus paper is stained with litmus (dye produced by lichens) and can either be red or blue. It is used to carry out a litmus test, to determine whether a substance is an acid or alkali.
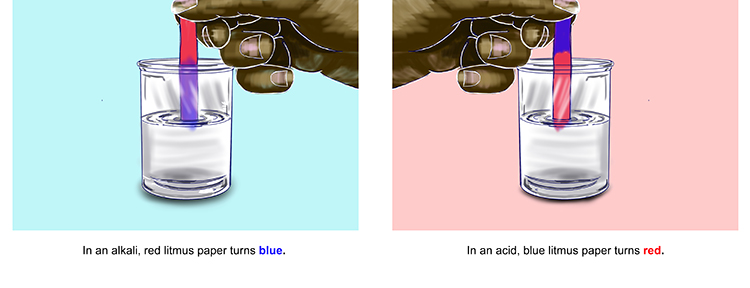
Red litmus paper changes to blue in alkalis, whilst blue litmus paper turns red in acids. In a neutral solution, the colour of both types of litmus paper does not change (i.e. red paper stays red and blue paper stays blue).
Universal indicator
Universal indicator is a combination of dyes that is green to begin with but changes colour when added to a solution. The colour it changes indicates not only if the substance is an acid or alkali, but its position on the pH scale.
Because universal indicator can turn a range of different colours, it is helpful in specifying the strength of an acid or alkali. As a result, it gives a more precise result than litmus paper, which only tells you if a solution is an acid or alkali.
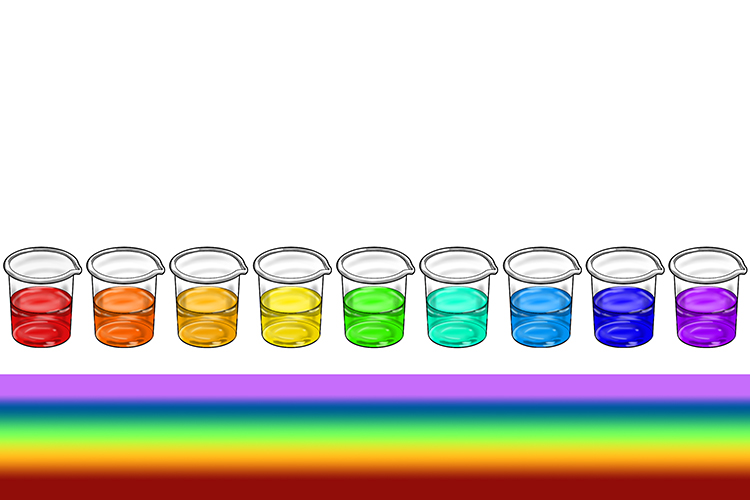
The colour changes of universal indicator correspond to the colours on the pH scale. It turns red, orange or yellow in acids, green in neutral solutions and blue or purple in alkalis.
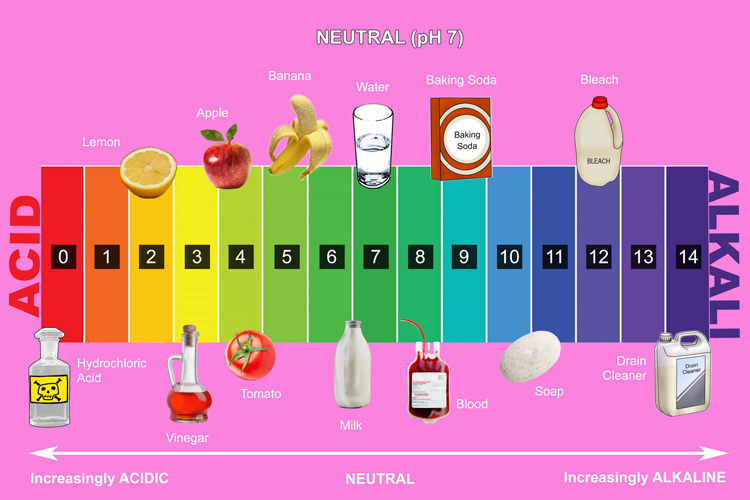
pH scale
The pH scale is used alongside universal indicator to determine the strength of an acid or alkali. It is numbered from 0 to 14, with each number associated with a particular colour. For example, if a solution turns light yellow, you know from checking the scale that it has a pH of between 3 and 4 and is mildly acidic.
The colours on the pH scale are the same as the colours of the rainbow.
Remember the old saying ‘Richard Of York Gave Battle In Vain’?
- Acids have a pH of between 0 and 7. Universal indicator turns red, orange or yellow
- Neutral substances have a pH of 7 and turn universal indicator green.
- Alkalis have a pH of between 7 and 14 and turn universal indicator dark green, blue or purple.