which energy levels fill up first, final thought
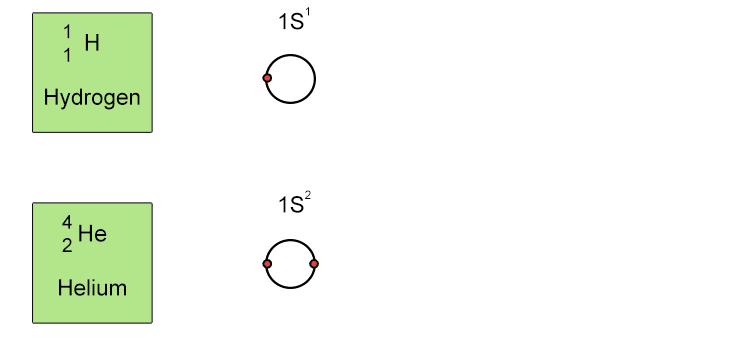
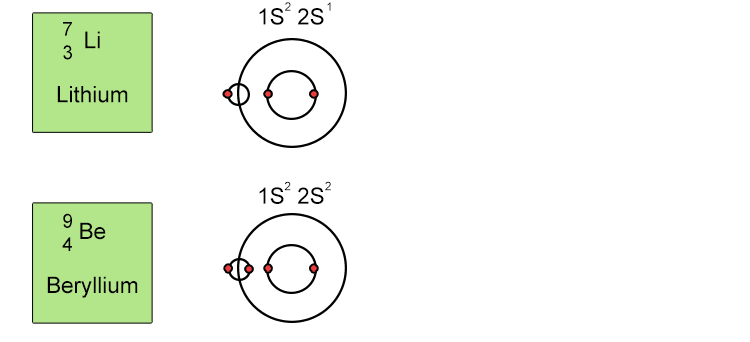
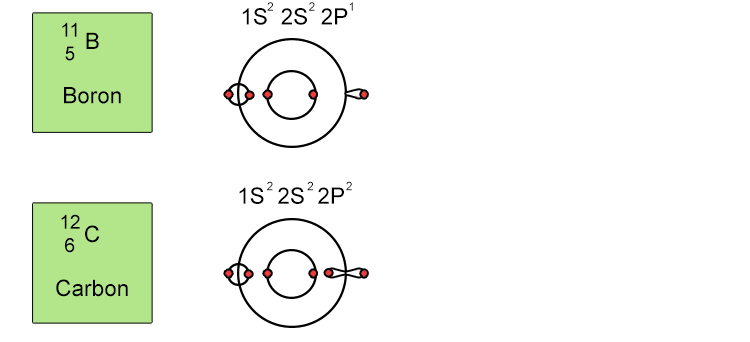
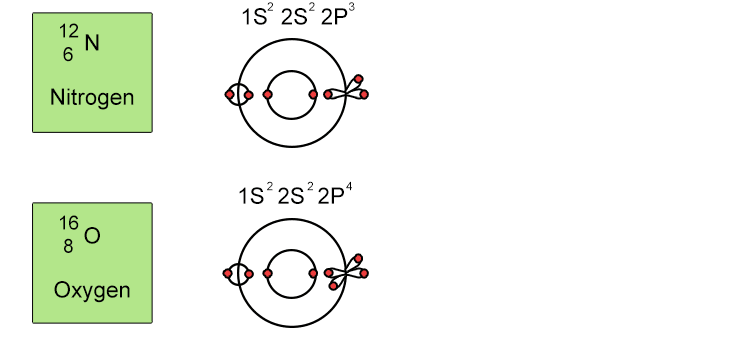
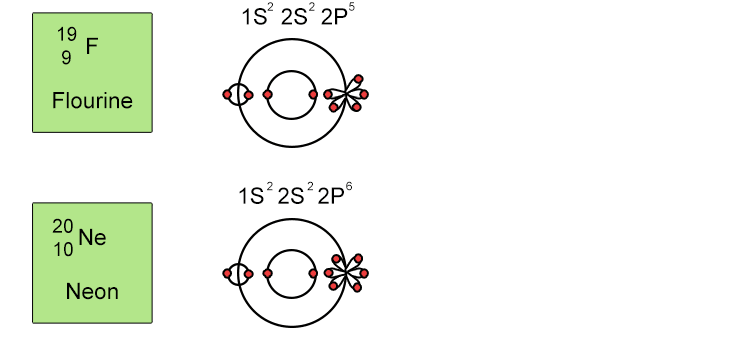
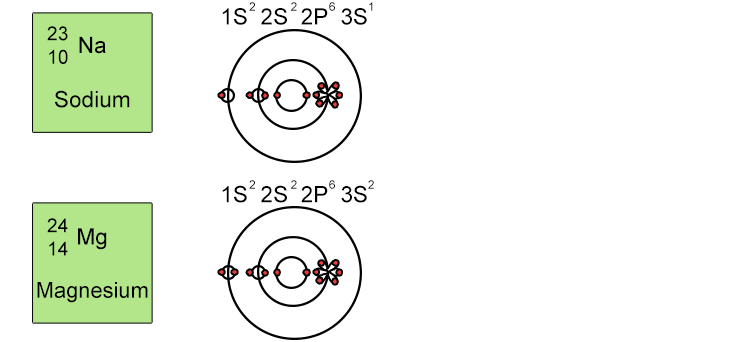
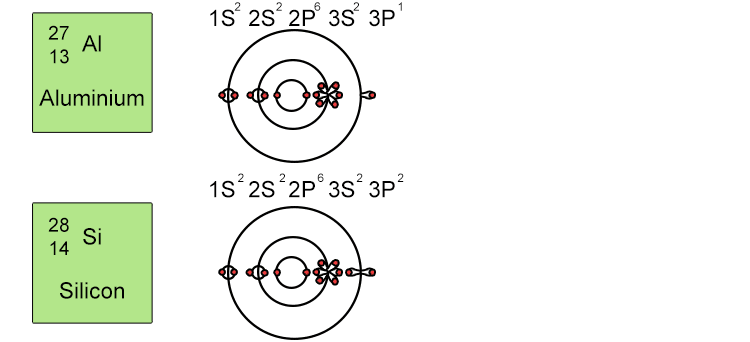
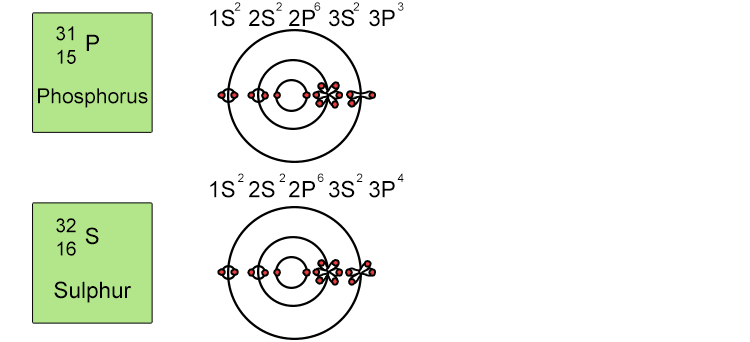
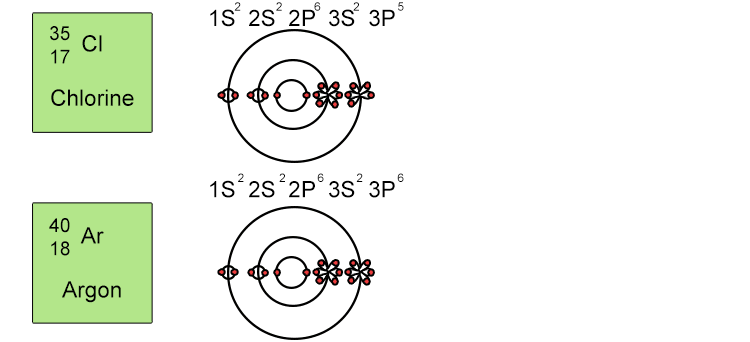
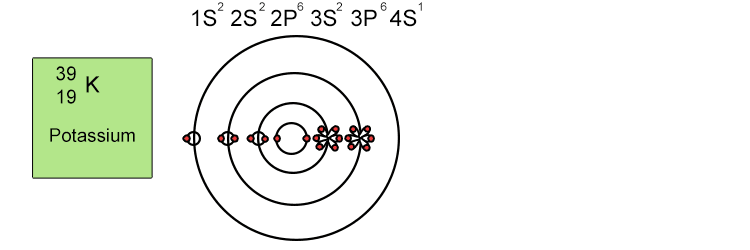
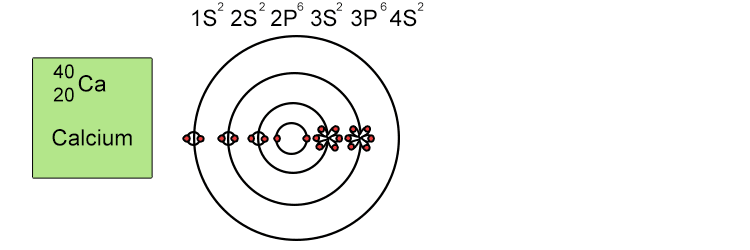
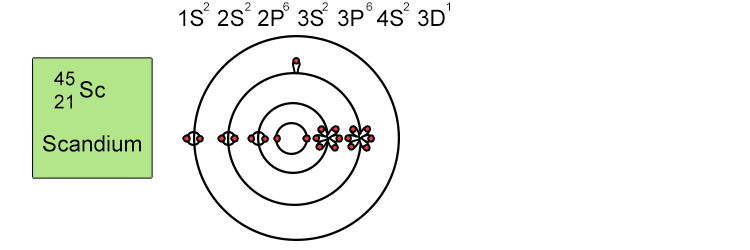
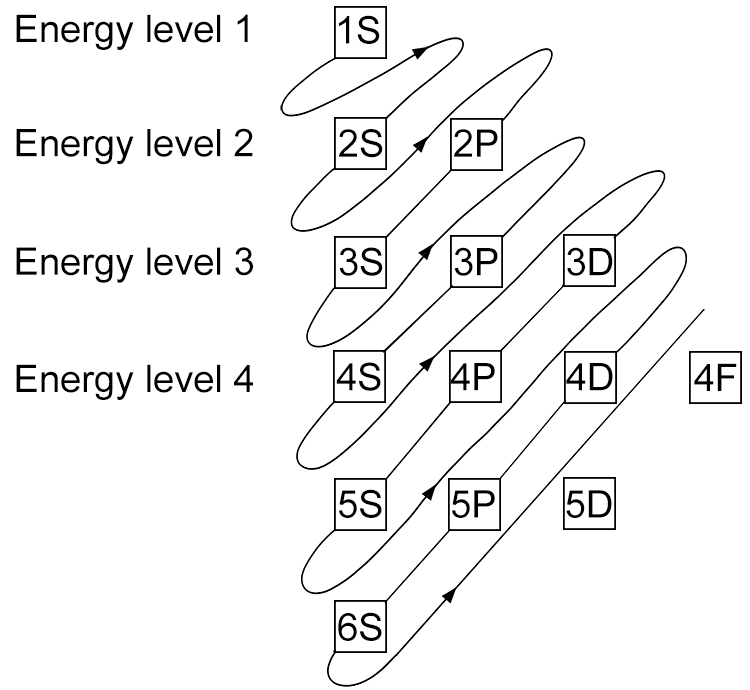
Unfortunately when the shells build up with electrons they don't always fill up each energy level fully and then go on to fill the next energy level. Below we show you how the electrons start to fill up each level. It's very logical up until level 3. At level 3 and after S and P sub shells are full of electrons, electrons start to fill up the S sub shell in level 4. When that's full, the electrons return to fill up the D sub shell in level 3. See below:

So, the sub shells fill up in the following order:
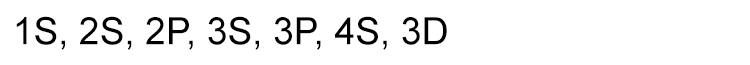
The order that electrons fill up each sub shell is shown in the following mnemonic chart:
This can be seen clearly on the following periodic chart:
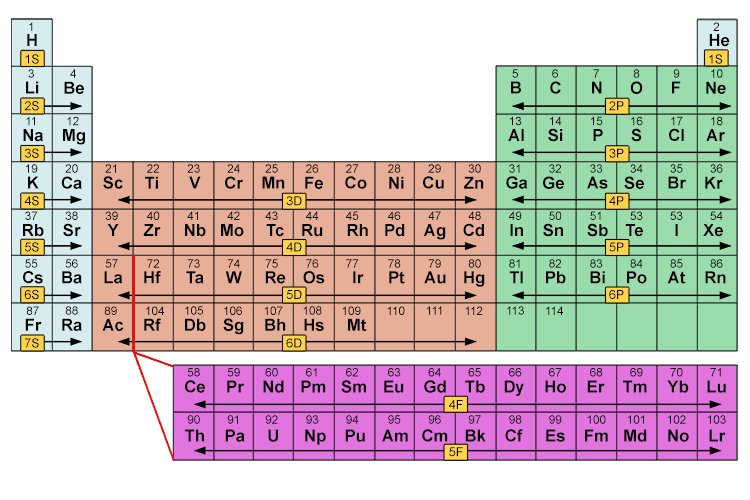
The reason sub shells do not fill up in a simple sequential order of sub shell letters S, P, D and F is because energy levels overlap between different principal shells.
Below is the order in which energy levels fill up: