Electrolytes in the body
Electrolyte = a liquid containing salts or minerals that conducts electricity
Electrolytes
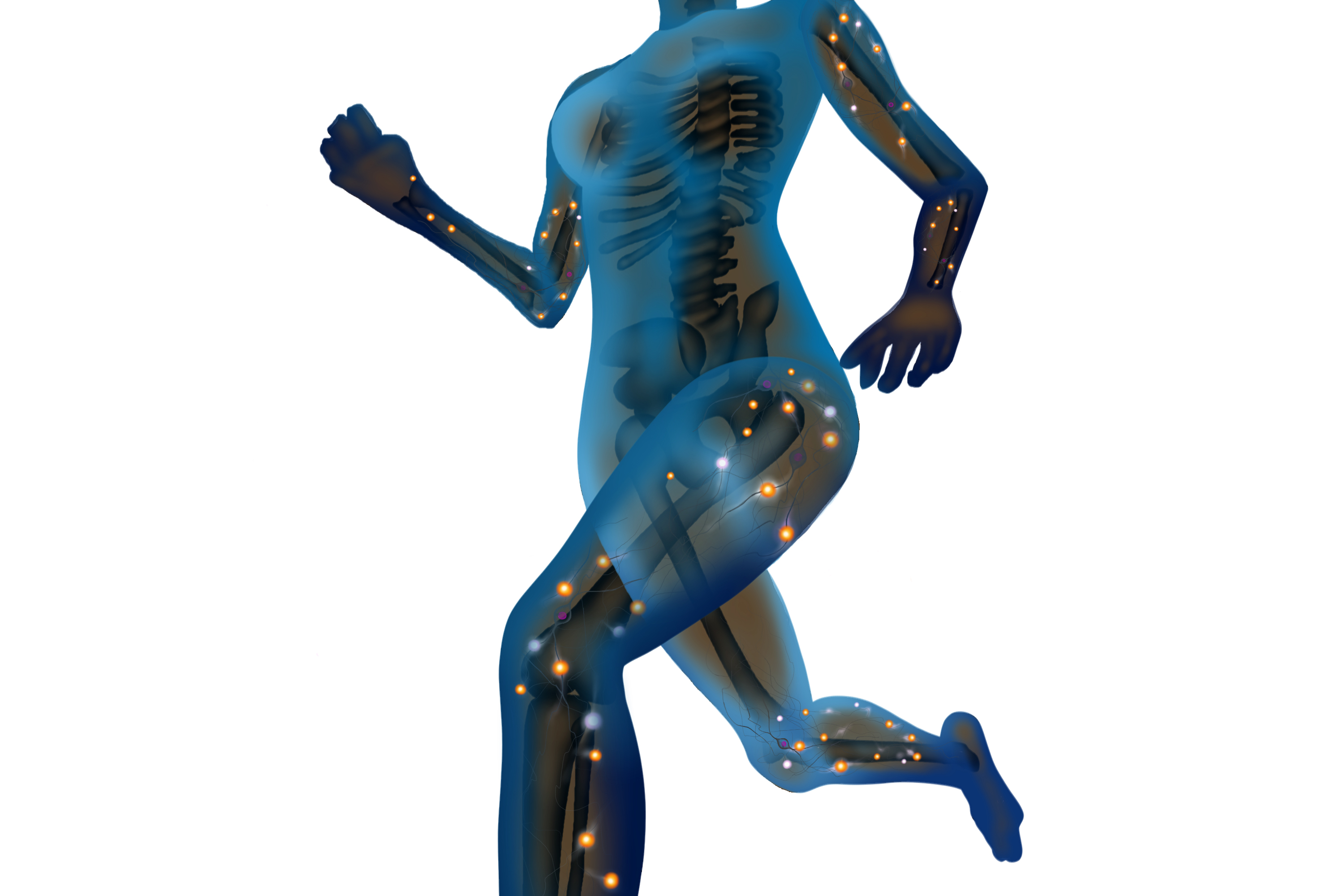
Athletes take electrolytes in sports drinks. This is because the body is a superhighway of electrical energy through cells, tissue and fluids.
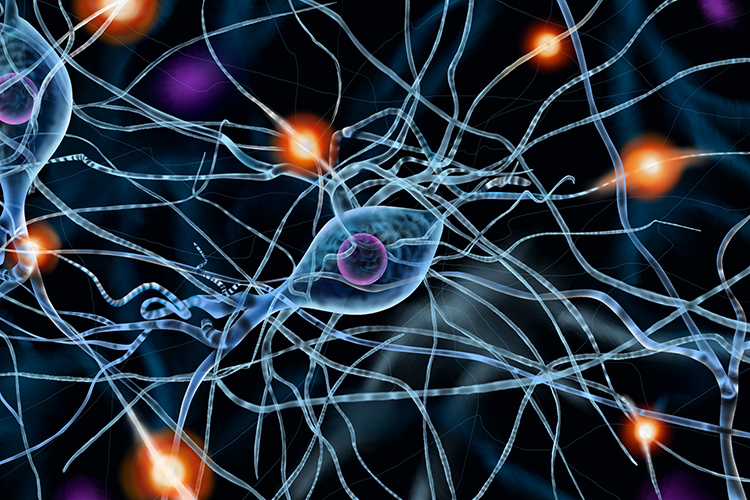
These electrical signals can carry electrical impulses around the body to send signals or messages to control muscle contraction, maintain water balance and maintain pH levels.
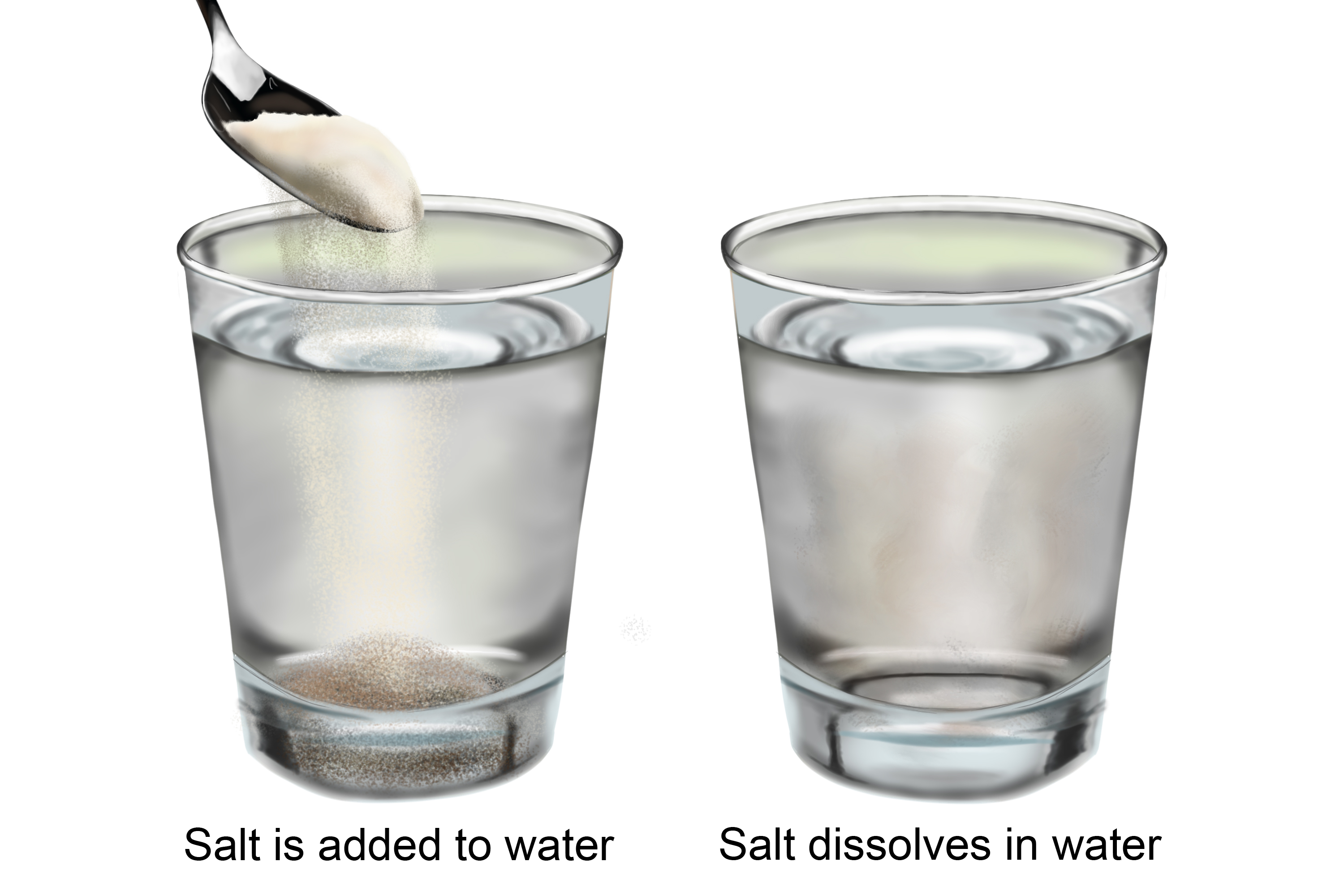
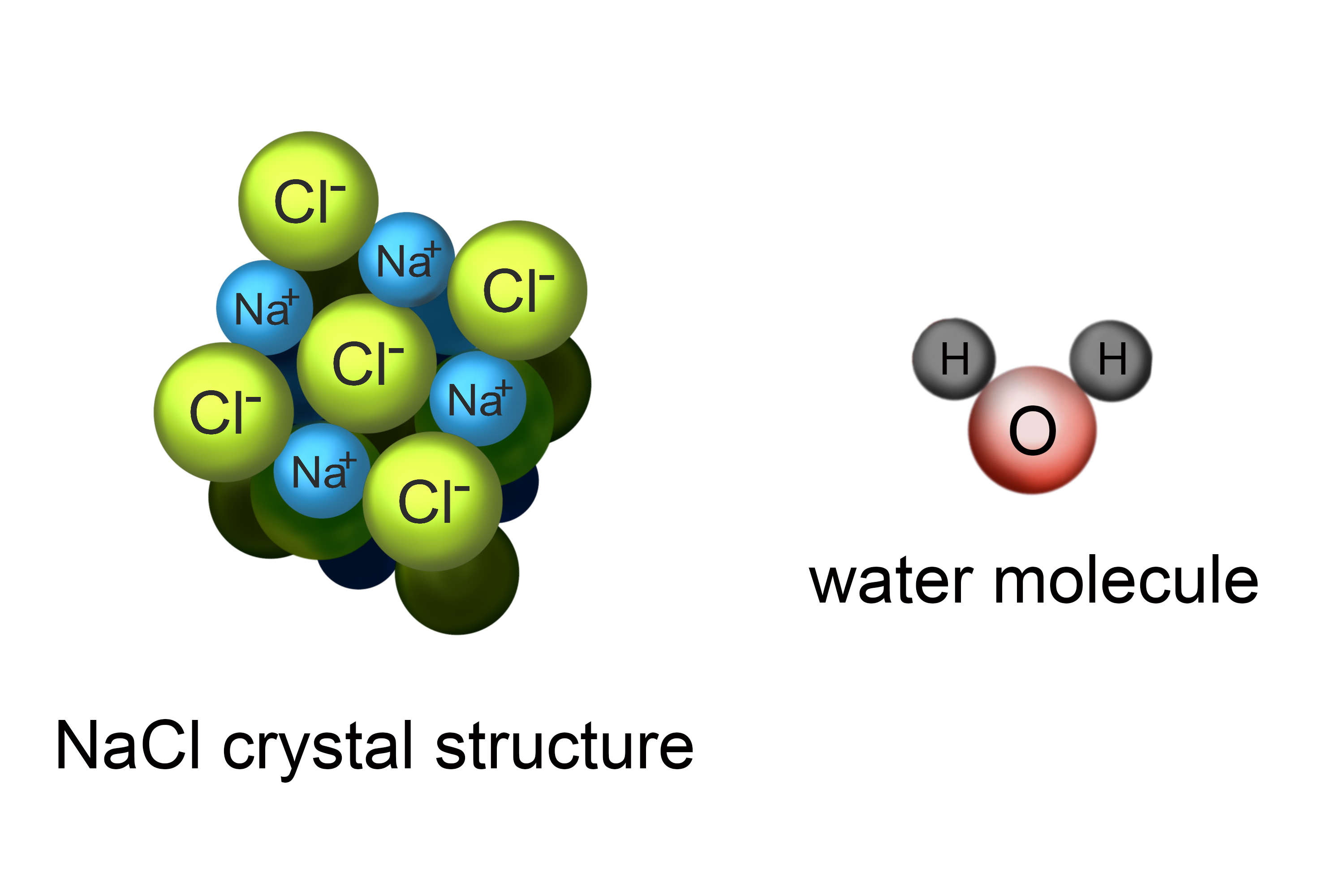
Your body needs electrolytes, which are chemicals that dissolve in fluid but can conduct electricity. For example, table salt (NaCl) will dissolve in water. The chemicals in salt – sodium and chlorine – will split apart and act independently in the water. This is called dissociation. Each part is called an ion - a negative chlorine ion (Cl-) and a positive sodium ion (Na+) - and is a good conductor of electricity.
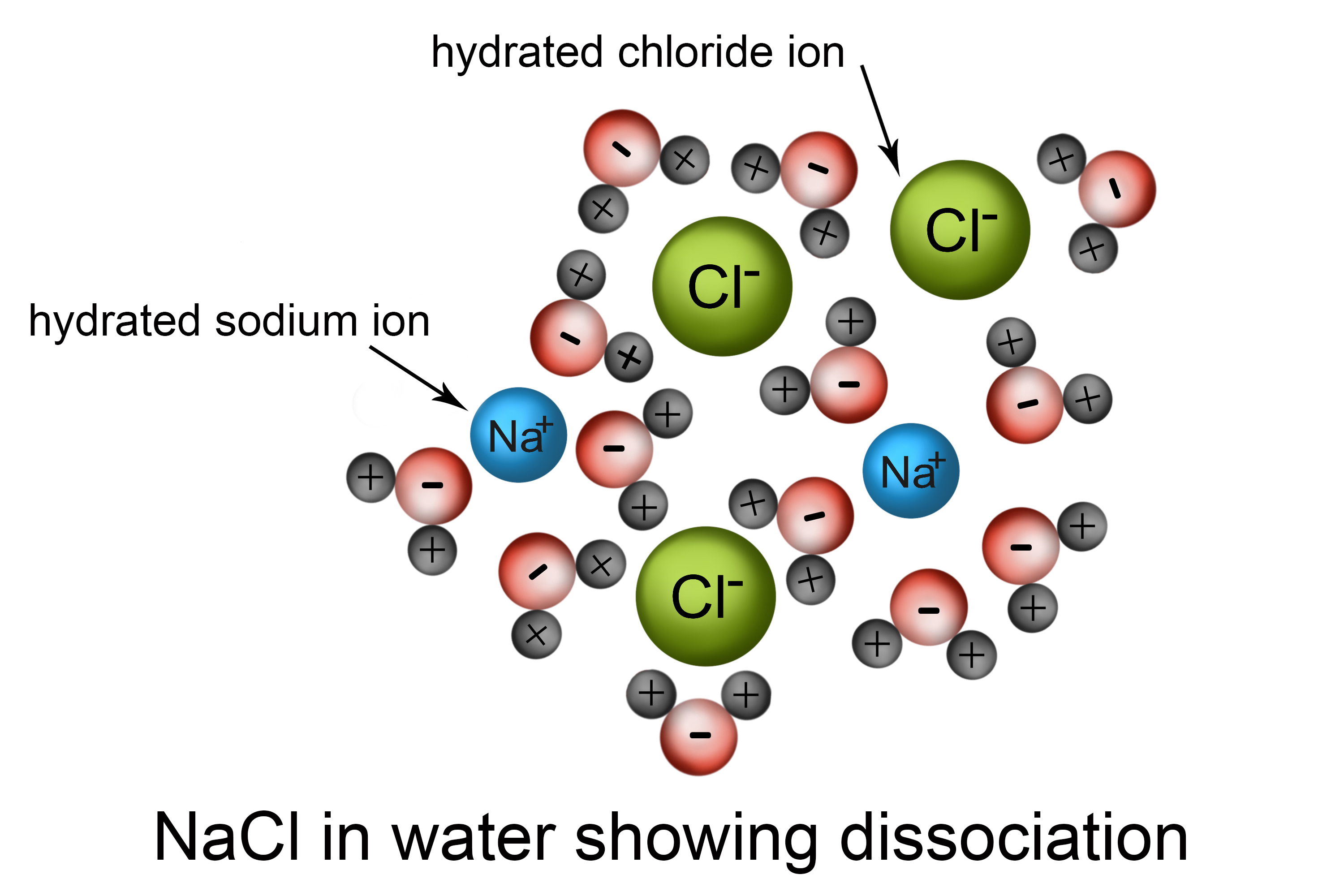
The body needs several electrolytes, each to serve a different function.
Sodium Na+
Chloride Cl-
Potassium K+
Magnesium Mg++
Calcium Ca++
Phosphate HPO4-
BUT the human body needs these to be at the correct balance. Too much or too little can be detrimental to your health.
The balance is vital for things such as nerve impulses (electrical signals), muscle function and pH level (acid/alkaline balance).
The kidneys maintain this balance.
Electrolyte is a liquid containing salts and minerals that conducts electricity.
The electrolyte is the liquid that can carry an electrical charge. The chemicals in it have dissociated.

Think of an electric troll light (electrolyte) swimming under water (liquid).

The electric troll light was being used to find an electric cable (conducts electricity) but all it could find was a giant salt pot (salts).




