Titration
Titration is a method for working out the concentration of a substance in a solution by gradually adding another substance until a reaction occurs.
We can use titration to work out the concentration of a quantity of acid or alkali (or base).

To remember titration, think of a classroom where the pupils are ties (titration). Some ties are concentrating harder than others (they are at different levels of concentration).
Titration
You can work out the concentration of a quantity of acid or alkali (or base) using titration.
There are many different titration experiments, this is just one example. In this experiment you'll need to use phenolphthalein indicator. Phenolphthalein has interesting properties which make it suitable for this experiment:
In its natural state and when mixed with an acid, it is colourless
When added to an alkaline solution, it suddenly turns pink
It is this immediate change of colour that explains why phenolphthalein is used for titrations instead of universal indicator. As universal indicator changes colour gradually, not suddenly, if it were used for this experiment, the point at which the solution becomes an alkali or acid would not be clear.
Finding the concentration of an acid
If you’re trying to work out the concentration of an acid, the acid goes in the conical flask and the alkali goes in the burette.
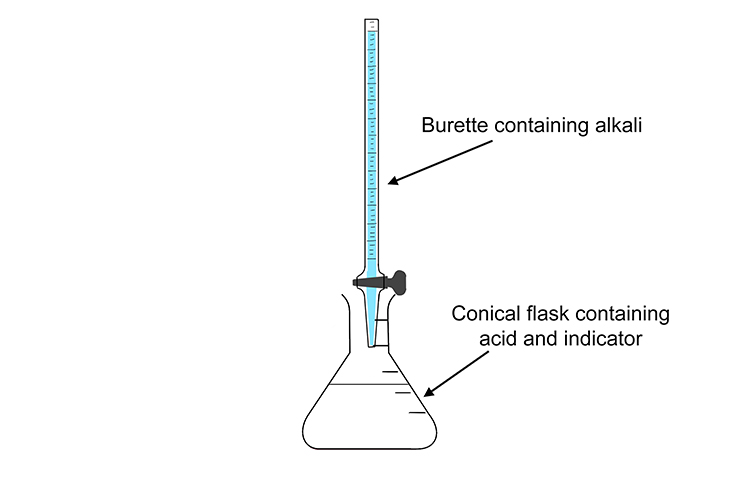
In the conical flask, when mixed with acid, the indicator is colourless. However, when enough alkali is released from the burette to cause the solution to become more alkaline than acidic or neutral, it immediately turns a strong pink colour.
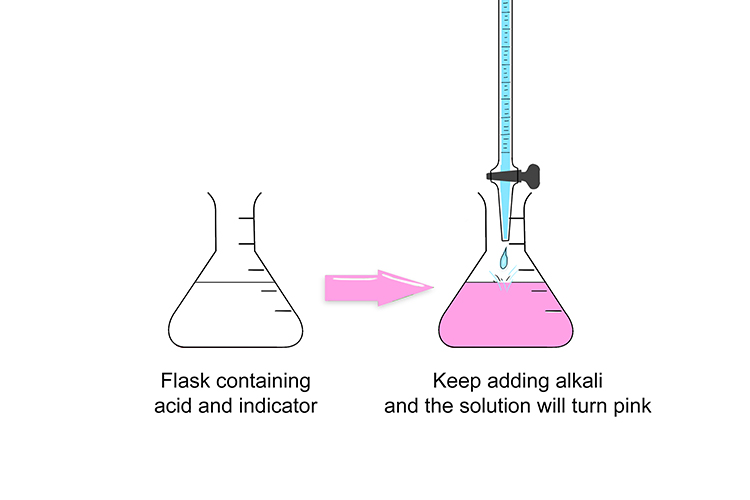
Slowly add the alkali into the flask. As soon as the solution turns pink, stop and make a note of how much alkali you have released from the burette.
Working out the concentration of an alkali
If you're trying to find out the concentration of the alkali, the titration equipment needs to be set up slightly differently; with the alkali and indicator in the conical flask and the acid in the burette.
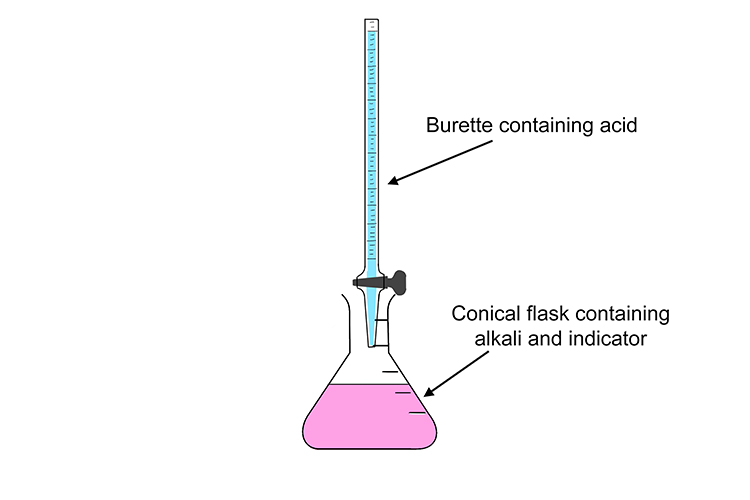
When mixed with an alkali in the conical flask, the indicator is pink. However, when enough acid is added and the solution neutralised, it suddenly becomes colourless.
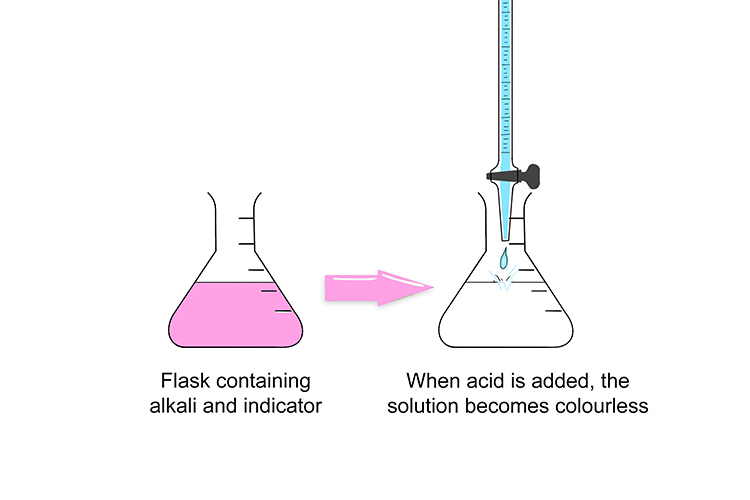
As soon as the solution changes colour, stop, and make a note of the amount of liquid you have used from the burette.
Repeat the experiment a couple of times and record how much acid or alkali you have released from the burette each time. Then calculate the mean of your results to find the average amount of liquid needed to change the colour of the solution.
You will then be able to calculate the concentration of the acid or alkali using the following method:-
You now know three pieces of information:
The amount of liquid you originally put in your conical flask
The amount of liquid you have used from your burette
The concentration of the liquid that was in the burette (it will say on the bottle that you poured it from!)
So, to find out the concentration of the liquid in the flask:-
Concentration of liquid in flask = Concentration of liquid in burette x Volume of liquid in burette
Volume of liquid in flask
WARNING: Your volume measurements MUST be in dm3 for this to work. To convert them from cm3 or millilitres (ml) to dm3, divide by 1000 (so 15cm3 = 0.015dm3).
Let’s run through an example:
We have 0.025dm3 (25ml) of sodium hydroxide (an alkali) of an unknown concentration in the conical flask, and we want to find out how concentrated it is.
We run a titration experiment (as described above) and, on average, we have to use 0.015dm3 of hydrochloric acid (HCl) to neutralise the sodium hydroxide (NaOH). We know that the hydrochloric acid’s concentration is 0.2M (M is the measurement for concentration – it stands for molar) because it says so on the side of the bottle we’re using! We can plug these numbers into the equation above. Here it is again, with a reminder of which liquid is where:
Concentration of NaOH (flask) = Concentration of HCl (burette) x Volume of HCl (burette)
Volume of NaOH (flask)
Concentration of NaOH (flask) = 0.2 x 0.015
0.025
Therefore we know that the concentration of the sodium hydroxide is 0.12M.




