Metals below copper in the reactivity series
Metals below copper in the reactivity series (silver, gold and platinum) won’t react with oxygen, even when heated.
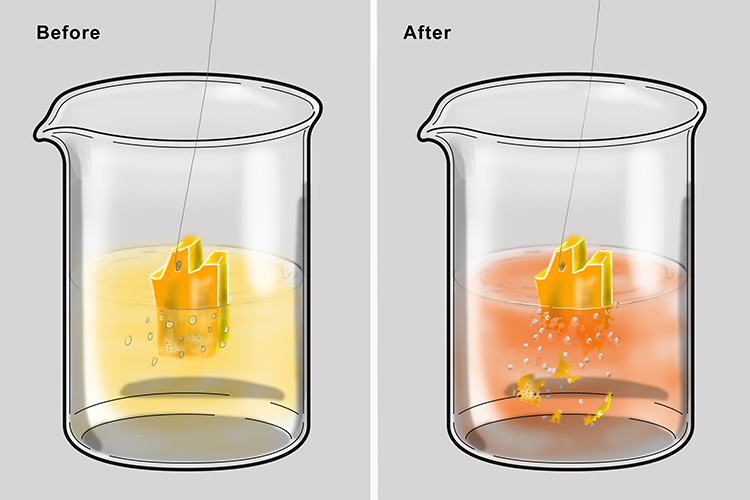
Gold
Gold is not very reactive. It can be extracted from its ore using electrolysis but is usually found in its pure state.
Gold dissolves in nitrohydrochloric acid (one of the strongest acids that exists) to make chloroauric acid.
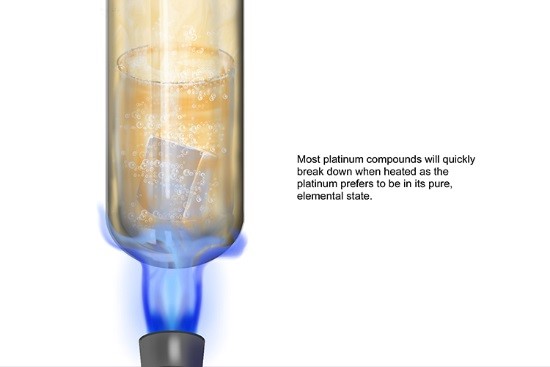
Platinum
Platinum is extremely unreactive. It’s not interested in forming bonds with other elements at all, and only does so if forced.
Platinum can be made to react with very strong acid (nitrohydrochloric acid), but only if it is heated. Essentially, you have to force it to react against its will!