Reactions with acid
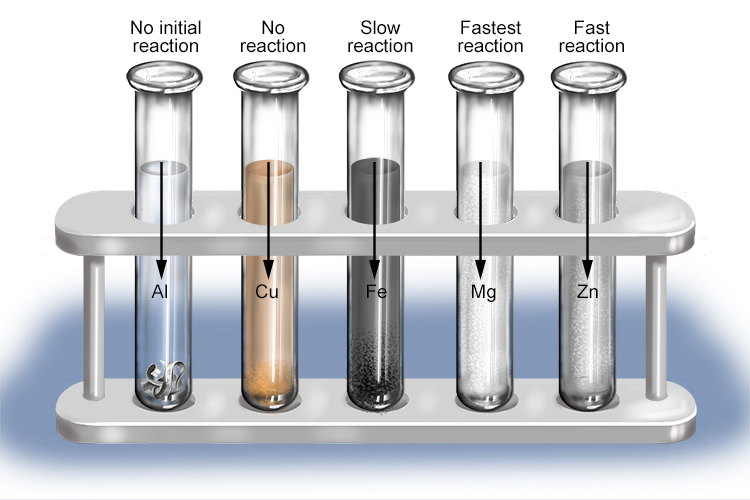
Group 1 metals (potassium, sodium and lithium) react violently even with dilute acids. The reaction will often produce enough heat to ignite the hydrogen gas given off.
Other metals produce less violent reactions with acids. The speed of these reactions and quantity of hydrogen produced can be used to arrange the metals in order of their reactivity, with aluminium being a notable exception (reaction of aluminium and hydrochloric acid).