Gay-Lussac's Law In Use
We know in Gay-Lussac's law
`P_1/T_1=P_2/T_2`
NOTE:
Volume remains constant
Also you know that if you put a sealed container in a fire it will make the pressure inside the canister increase.
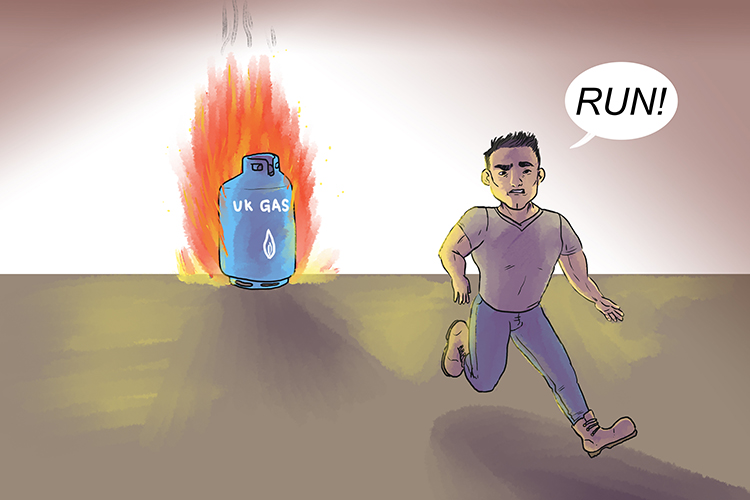
This is Gay-Lussac's law
`P_1` and `T_1` refer to the pressure and temperature before you throw the container in the fire and `P_2` and `T_2` refer to the pressure and temperature after you throw the container in the fire.
Increasing the temperature of the gas increases the pressure in the container.
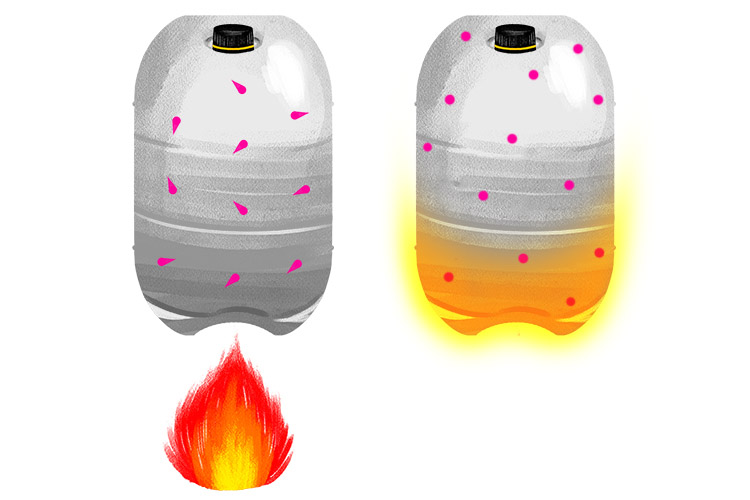
Increasing the temperature of a gas speeds up the molecules and they have more energy (kinetic energy) to hit and push the side of the container. The molecules will exert more pressure.
NOTE 1:
The standard unit used to measure pressure is `Pa` (Pascals)
The standard unit used to measure temperature is `K` (Kelvin)
NOTE 2:
You can use other units for pressure such as pounds per square inch or `mm` of mercury (mm Hg) or atm (atmospheres) as long as you use the same units either side of the equation but you must use Kelvin for temperature.
NOTE 3:
Never forget to convert temperature to Kelvin
Temperature in Kelvin `=` Temperature in degrees Celsius `(\ ^(circ)C)+273`
You must always use temperature measured in Kelvin in any gas law equation.
Examples
1. A `30\ \l` sample of nitrogen inside a rigid, metal container at `20^circC` is placed inside an oven whose temperature is `50^circC`. The pressure inside the container at `20^circC` was at `3.00 atm`. What is the pressure of the nitrogen after its temperature is increased to `50^circC` ?
1. A `30\ \l` sample of nitrogen inside a rigid, metal container at `20^circC` is placed inside an oven whose temperature is `50^circC`. The pressure inside the container at `20^circC` was at `3.00 atm`. What is the pressure of the nitrogen after its temperature is increased to `50^circC` ?
`P_1/T_1=P_2/T_2`
`P_1=3atm`
`P_2=?`
`T_1=20^circC+273=293K`
`T_2=50^circC+273=323K`
Therefore `3/293=P_2/323`
`P_2=(3xx323)/293`
`P_2=3.31atm` (to three significant figures)
2. If a gas in a closed container is pressurized from `15` atmospheres to `16` atmospheres and its original temperature was `25^circC`, what would the final temperature of the gas be in degrees Celsius?
2. If a gas in a closed container is pressurized from `15` atmospheres to `16` atmospheres and its original temperature was `25^circC`, what would the final temperature of the gas be in degrees Celsius?
`P_1/T_1=P_2/T_2`
`P_1=15atm`
`P_2=16atm`
`T_1=25^circC+273=298K`
`T_2=?`
Therefore `15/298=16/T_2`
`T_2=(16xx298)/15`
`T_2=317.9K`
But don't forget to convert back from Kelvin to degrees Celsius
Therefore `T_2=317.9-273=44.9^circC`
3. The temperature of a sample of gas in a steel container at `30\ \kPa` is increased from `-100^circC` to `1.00xx10^3\ \^circC`. What is the final pressure inside the tank.
3. The temperature of a sample of gas in a steel container at `30\ \kPa` is increased from `-100^circC` to `1.00xx10^3\ \^circC`. What is the final pressure inside the tank.
`P_1/T_1=P_2/T_2`
`P_1=30\ \kPa`
`P_2=?`
`T_1=-100^circC+273=173K`
`T_2=1.00xx10^3\ \^circC=1000^circC+273=1273K`
Therefore `P_2=(P_1xxT_2)/T_1`
`P_2=(30xx1273)/173`
`P_2=221\ \kPa` to three significant figures




