Ions
Ions are created when a neutral atom loses or gains electrons.
.b1de29e.jpg)
An ion is an atom or molecule that carries an electric charge.
Ions are identified with the sign and size of their charge.
Negatively charged ions are called anions.
Positively charged ions are called cations.
Examples of anions:
Br ⁻ = Bromine atom which has gained an electron
N³⁻ = Nitrogen atom which has gained 3 electrons
Examples of cations:
Na⁺ = Sodium atom which has lost an electron
Fe³⁺ = Iron atom which has lost 3 electrons
Example 1
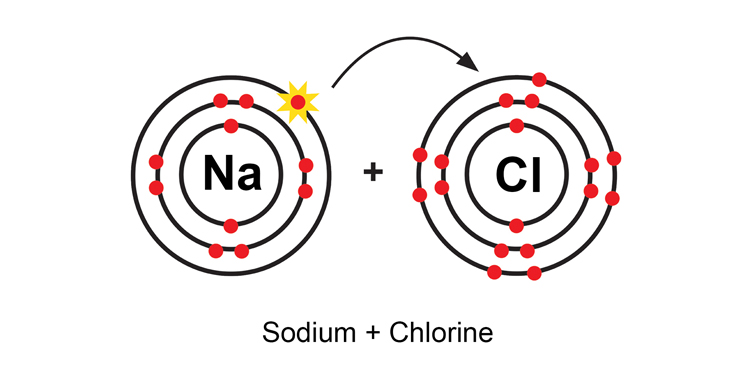
When a sodium atom and chlorine atom meet, the sodium atom loses an electron and the chlorine atom gains the electron, to give:
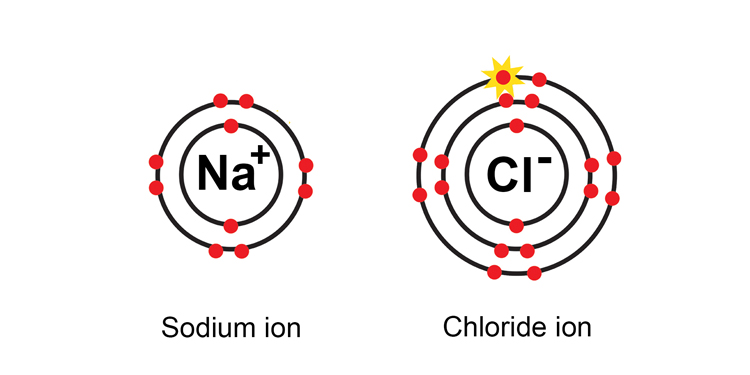
Note: When chlorine gains an electron to become an ion, it is known as a chloride ion not a chlorine ion.
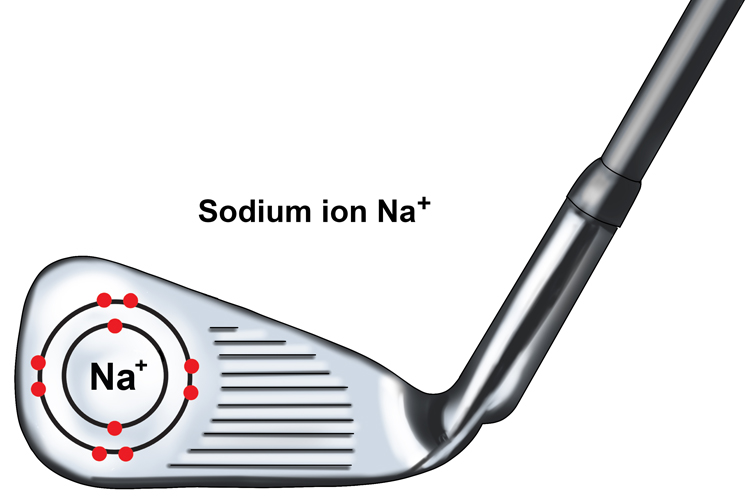
The sodium ion is said to be a one + ion or Na+ ion as it has lost one negatively charged electron.
The chlorine atom becomes a one – ve chloride ion or Cl- as it has gained one negatively charged electron.
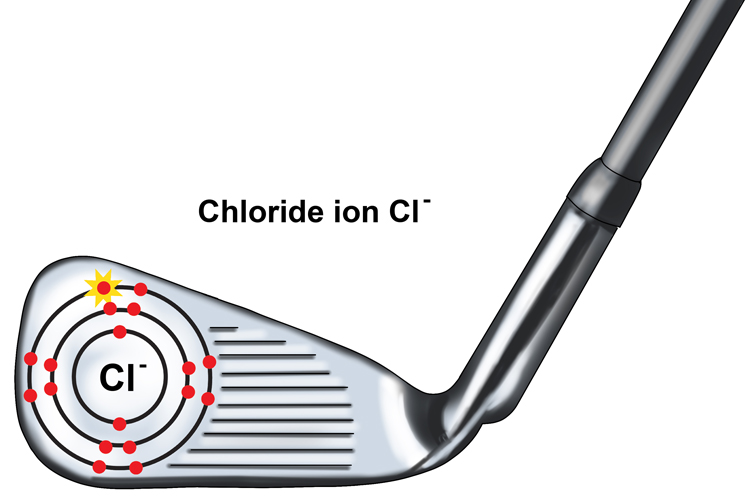
The positive and negative charges of these ions attract one another and this forms an ionic bond to make the compound sodium chloride or NaCl.
Example 2
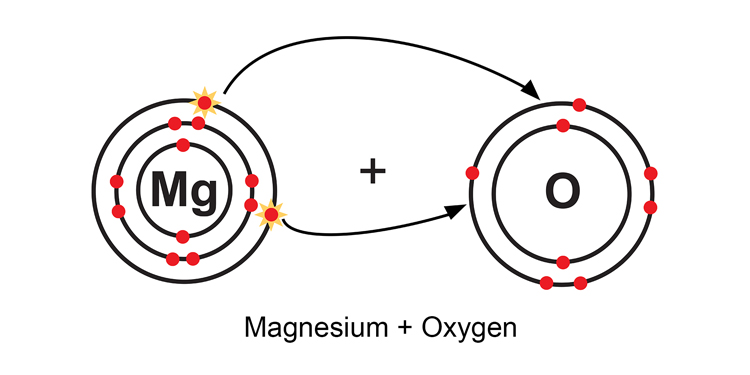
When a magnesium atom and oxygen atom meet, the magnesium atom loses two electrons and the oxygen atom gains the two electrons, to give:
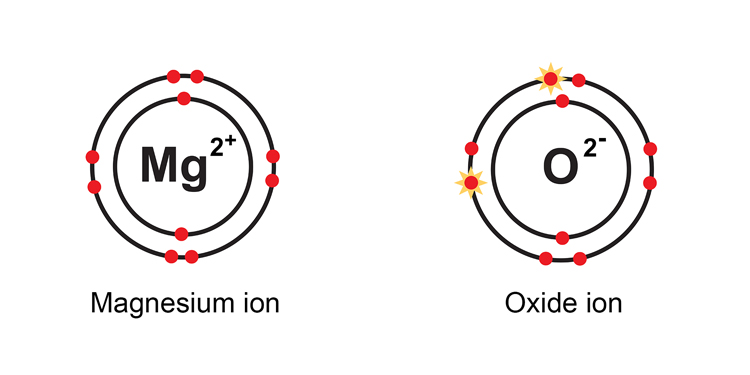
Note: When oxygen gains electrons to become an ion, it is known as an oxide ion not an oxygen ion.
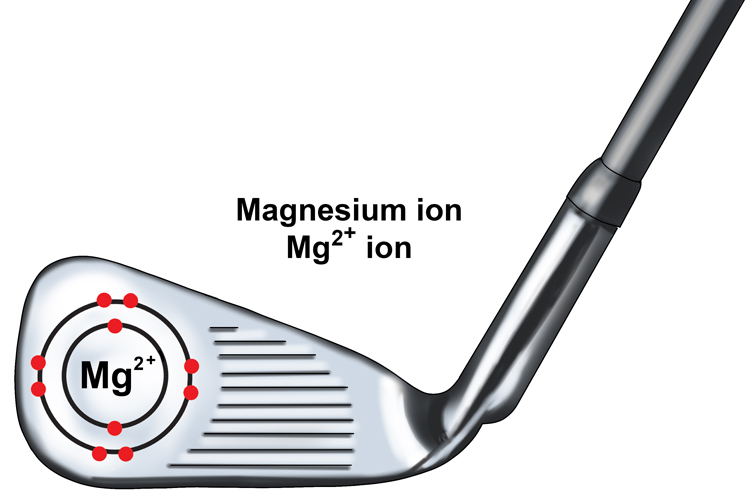
The magnesium is said to be a two + ion or Mg2+ ion as it has lost two negatively charged electrons.
The oxygen atom becomes a two – ve oxide ion or O2- as it has gained two negatively charged electrons.
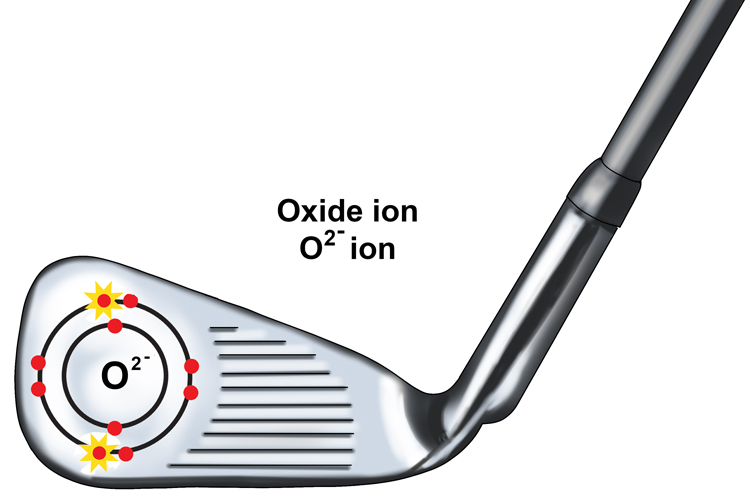
The positive and negative charges of these ions attract one another, forming an ionic bond to make the compound magnesium oxide or MgO.




