ion – a charged atom
(pronounced ayh-on)
Note 1: An ion is a charged molecule too.
Note 2: Charged means the atom has an unequal number of protons and electrons giving it an overall electric charge.
To remember the meaning of ion, use the following mnemonic:
The iron (ion) charged at the atom bomb.
An ion is an atom or molecule (group of atoms) that has an electric charge because it has gained or lost electrons. If it loses electrons, it becomes a positively charged ion called a cation. If it gains electrons, it becomes a negatively charged ion called an anion.
To remember cations and anions link here.
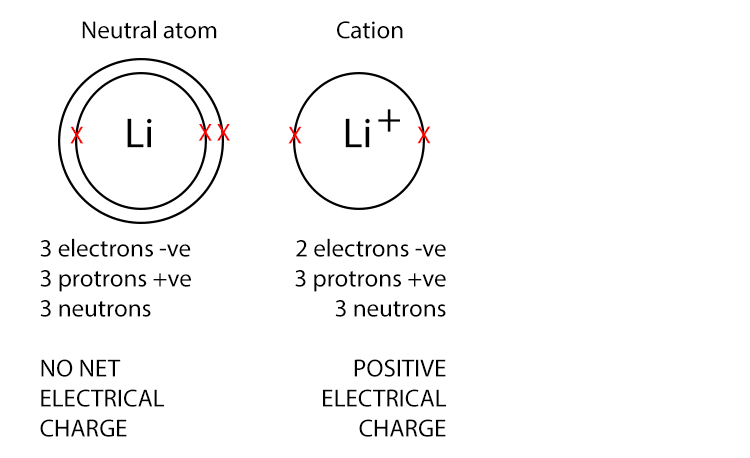
Cations have a positive charge because they have more protons than electrons. Metal atoms typically lose electrons to form cations.
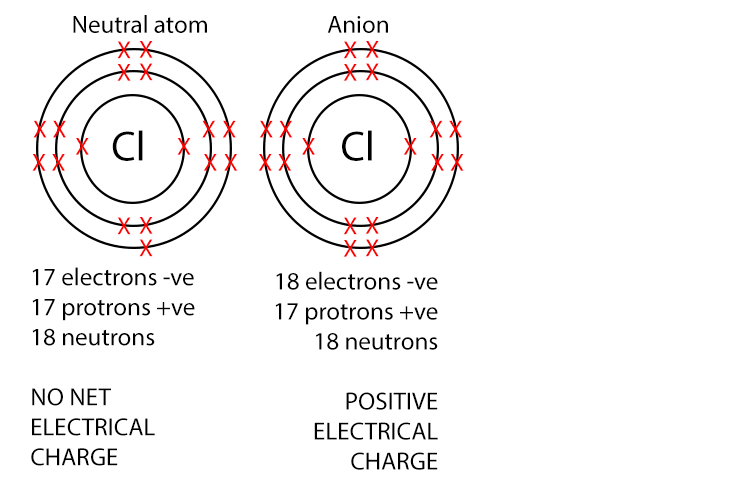
Anions have negative charge because they have more electrons than protons. Non-metals atoms typically gain electrons to form anions.




