Displacement from oxide or solution
A displacement reaction is where a more reactive metal takes the place of a less reactive metal.
There are two types of displacement reaction:
1. Displacement from an oxide (solid state competition reaction)
2. Displacement from a solution (competition reaction in aqueous solution)
Displacement from oxide
In a metal and metal oxide displacement reaction, the more reactive metal will take the oxygen atoms to form a metal oxide, leaving behind the less reactive metal.
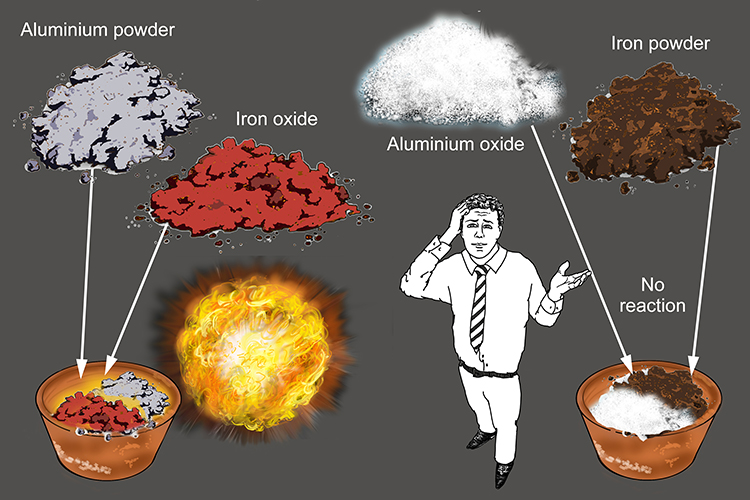
If the metal oxide you start with is formed from a more reactive metal than the metal you add, no reaction will occur.
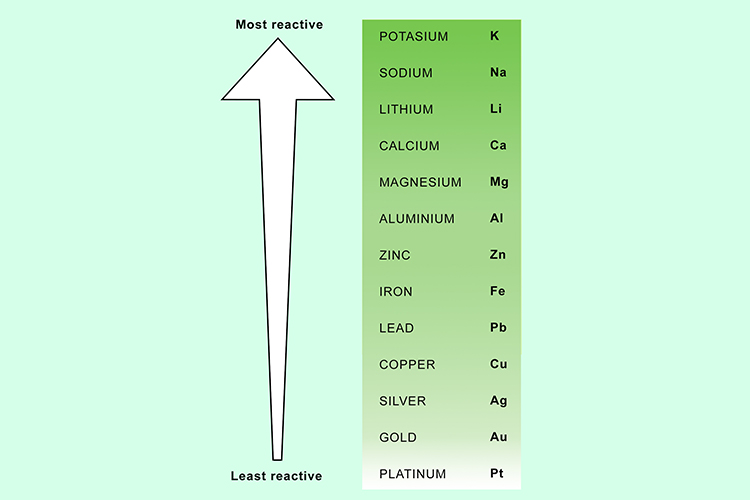
You can now predict which metal will win by using the reactivity series of metals.
NOTE: This can be used as an alternative method of testing to put the reactivity series in the correct order.
Example 1
| Aluminium oxide | + | Iron | → | No reaction |
But
| Aluminium | + | Iron oxide | → | Aluminium oxide | + | Iron |
For more details, see applications of oxide displacement reactions.
Example 2
| Iron oxide | + | Copper | → | No reaction |
But
| Iron | + | Copper oxide | → | Iron oxide | + | Copper |
Iron and copper oxide displacement reaction
Iron + copper oxide → Iron oxide + Copper
2Fe + 3CuO → Fe2O3 + 3Cu
When iron reacts with copper oxide, the iron will take the oxygen atoms from the copper oxide to form iron oxide, as iron is more reactive than copper.
However, iron oxide will not react with copper, as copper is less reactive than iron.




