Extracting iron
Carbon can be used to extract the iron from iron ore (haematite/ iron oxide Fe2O3). This process takes place in a blast furnace.
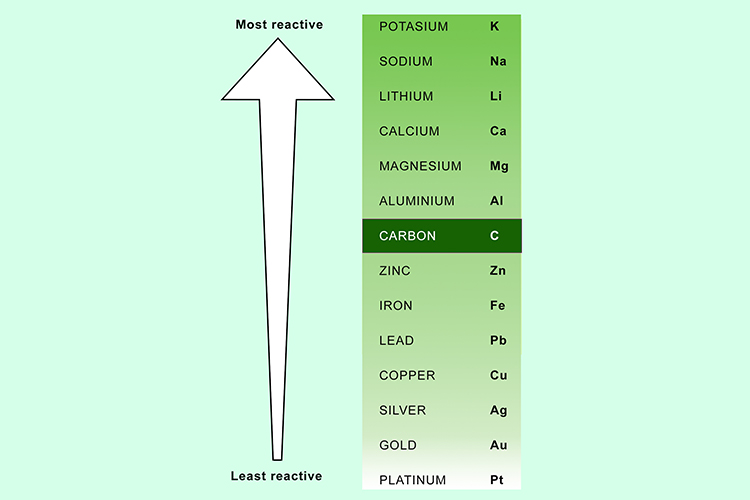
Because carbon is more reactive than iron, it will remove the oxygen from the iron oxide.





Carbon can be used to extract the iron from iron ore (haematite/ iron oxide Fe2O3). This process takes place in a blast furnace.

Because carbon is more reactive than iron, it will remove the oxygen from the iron oxide.