Displacement from solution
If a metal and metal solution react, the more reactive metal will displace the less reactive metal from solution.
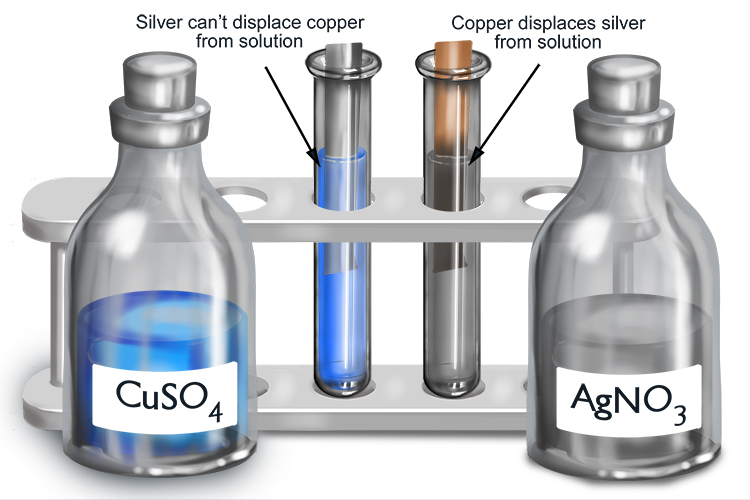
If the metal in solution you start with is formed from a more reactive metal than the metal to be added, no reaction will occur.
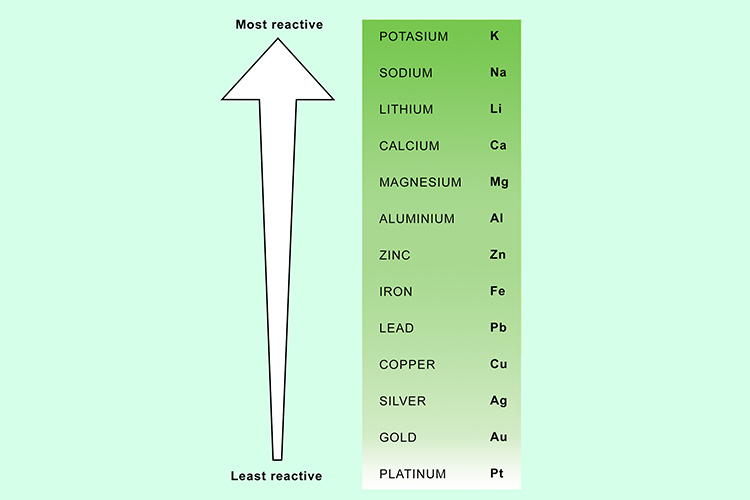
You can now predict which metal will win by using the reactivity series of metals.
Note: This can be used as an alternative method of testing to put the reactivity series in the correct order.
Example 1
| Zinc nitrate | + | Lead | → | No reaction |
But
| Lead nitrate | + | Zinc | → | Zinc nitrate | + | Lead |
Example 2
| Magnesium nitrate | + | Lead | → | No reaction |
But
| Lead nitrate | + | Magnesium | → | Magnesium nitrate | + | Lead |
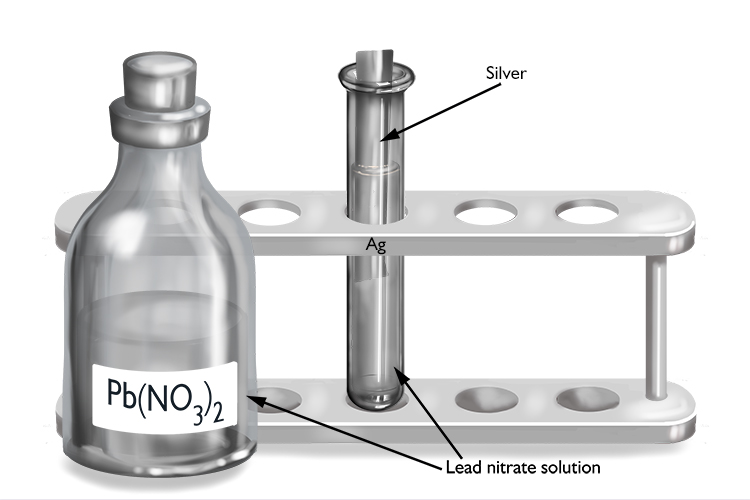
Lead nitrate and silver
| Lead nitrate | + | Silver | → | No reaction |
| Pb(NO3)2 | + | Ag | → | No reaction |
As no reaction occurs, lead must be more reactive than silver.
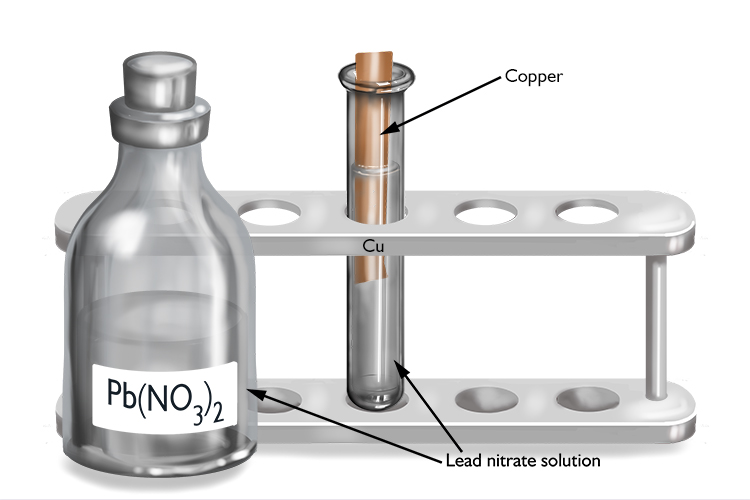
Lead nitrate and copper
| Lead nitrate | + | Copper | → | No reaction |
| Pb(NO3)2 | + | Cu | → | No reaction |
As no reaction occurs, lead must be more reactive than copper.
Lead nitrate and zinc
| Lead nitrate | + | Zinc | → | Zinc nitrate | + | Lead |
| Pb(NO3)2 | + | Zn | → | Zn(NO3)2 | + | Pb |
The zinc displaces the lead from solution to form zinc nitrate, so zinc must be more reactive than lead.
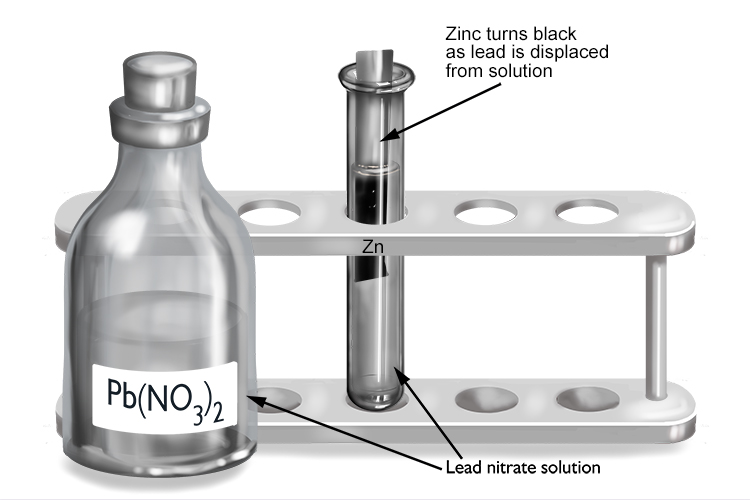
The zinc turns black as it becomes coated in a layer of lead that’s been displaced from the lead nitrate solution.
Lead nitrate and magnesium
| Lead nitrate | + | Magnesium | → | Magnesium nitrate | + | Lead |
| Pb(NO3)2 | + | Mg | → | Mg(NO3)2 | + | Pb |
The magnesium displaces the lead from solution to form magnesium nitrate, so magnesium must be more reactive than lead.
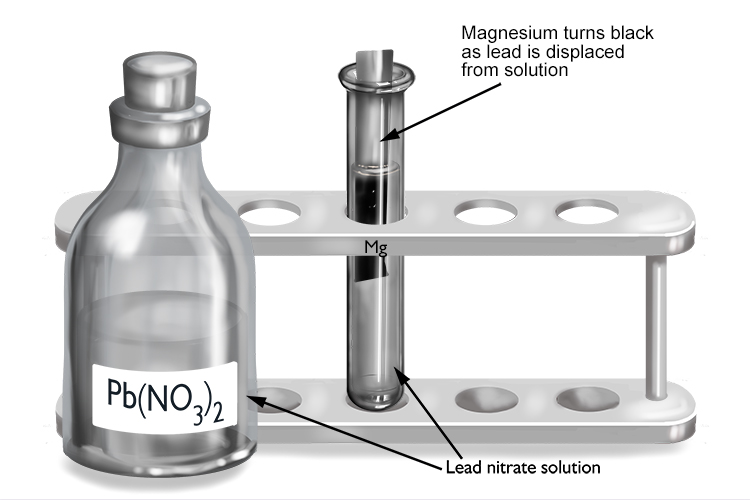
The magnesium turns black as it becomes coated in a layer of lead that’s been displaced from the lead nitrate solution.
Zinc sulphate and magnesium
| Zinc sulphate | + | Magnesium | → | Magnesium sulphate | + | Zinc |
| ZnSO4 | + | Mg | → | MgSO4 | + | Zn |
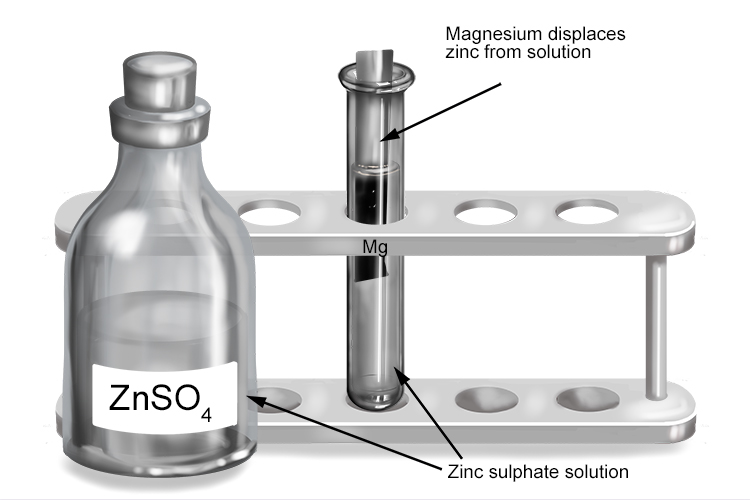
The more reactive magnesium displaces the less reactive zinc from the solution to form magnesium sulphate.
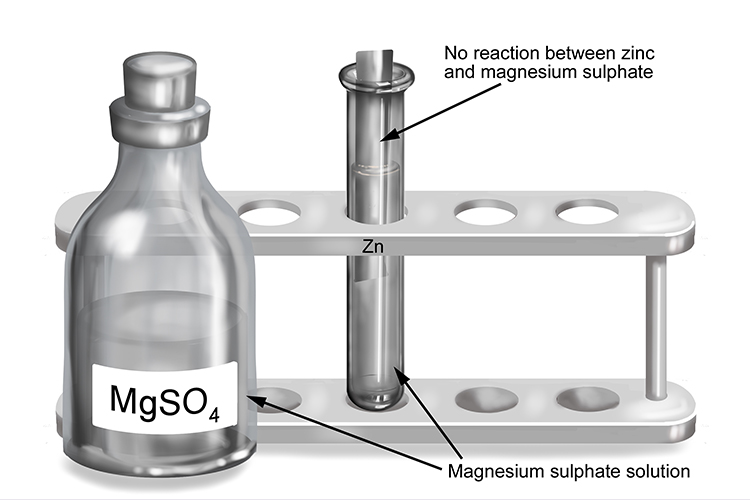
Magnesium sulphate and zinc
| Magnesium sulphate | + | Zinc | → | No reaction |
| MgSO4 | + | Zn | → | No reaction |
The less reactive zinc will not react with the solution which contains the more reactive magnesium.