Extracting metals from their ores
The reactivity series of metals is important when it comes to extracting a metal from its ore. The higher a metal is in the reactivity series the harder it is to extract from its ore.
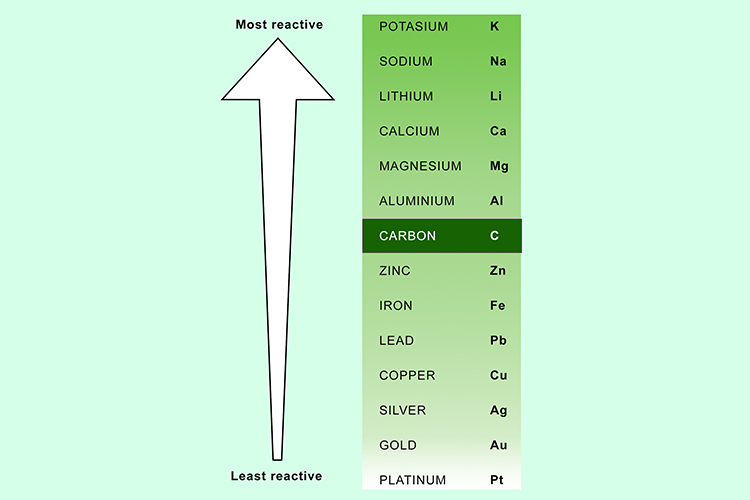
Carbon is not a metal but included within the reactivity series as it affects how the metals can be extracted.
The most reactive metals (ones above carbon) in the series have to be extracted by electrolysis. Metals below carbon can be extracted in a reduction reaction with carbon.
.jpg)
Carbon will out compete a metal below it in the reactivity series for an ore’s oxygen. This is called a reduction reaction as it reduces the metal oxide to a metal element.
(Although metal competing with metal oxide can also be considered a “reduction method”)
(See reduction and oxidation for more info on these reactions)
Remember OIL RIG
Oxidation Is Loss Reduction Is Gain
With regard to electrons.




