Distillation: an explanation
If a solution is made up of constituents that have different boiling points, we can separate these constituents by vaporising (boiling off) the one with the lower boiling point then condensing the vapour.
Example – Desalination (salt and water solution)
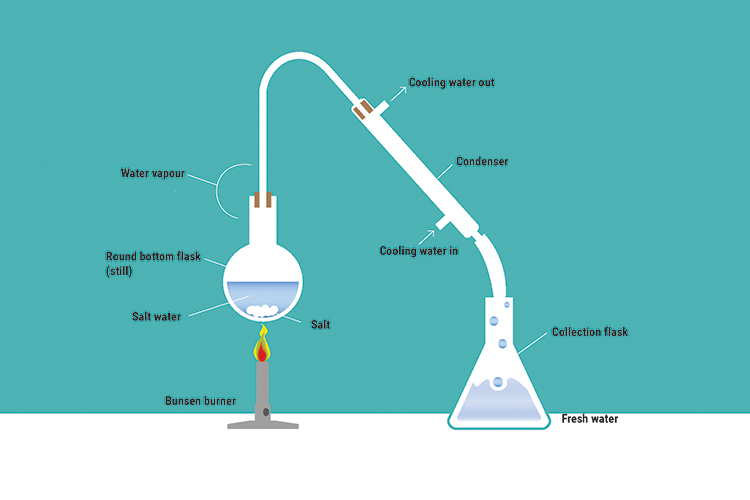
The water is boiled until it evaporates and leaves the still as steam. The steam is then condensed and collected in the flask, and the salt is left in the still.
I.e. The solution is separated by vaporising and condensing.




