Fractionating column for crude oil: condensing points and temperature
Within the fractionating column, it’s hot at the bottom of the column and gets gradually cooler towards the top. Smaller molecules require less energy (heat) than larger molecules to change them from liquid to vapour. The smaller molecules condense (change back to liquid) further up the column. This is how the different sized molecules (fractions) are separated.
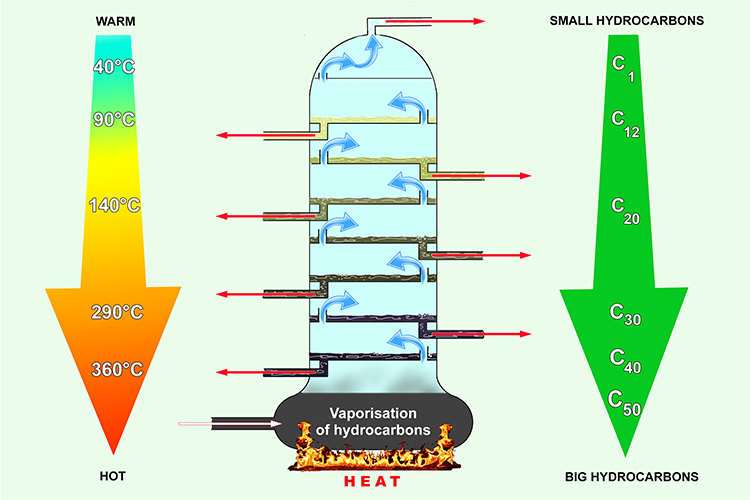
From the top to the bottom, the hydrocarbon molecules get bigger as the temperature of the column gets hotter.
For more information on hydrocarbons and to see what small C1 and larger hydrocarbon molecules look like, follow the link to our hydrocarbons page.
But as a quick summary, see the following:
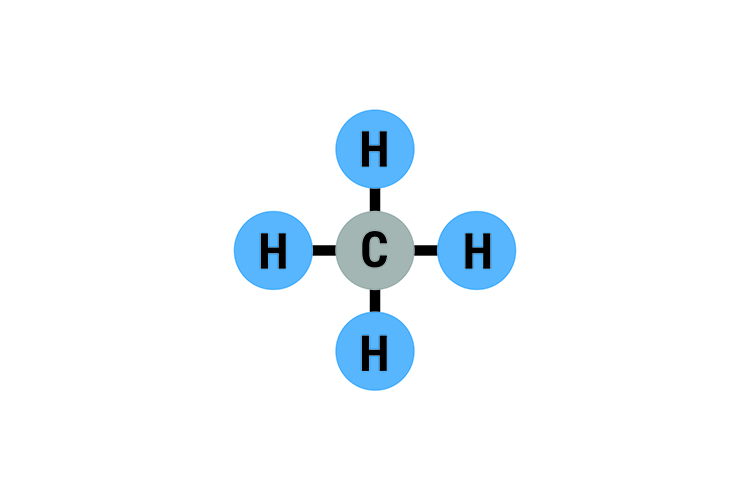
Methane CH4: Boiling point -162°C (-259°F)
Note: Methane is the main constituent of natural gas.
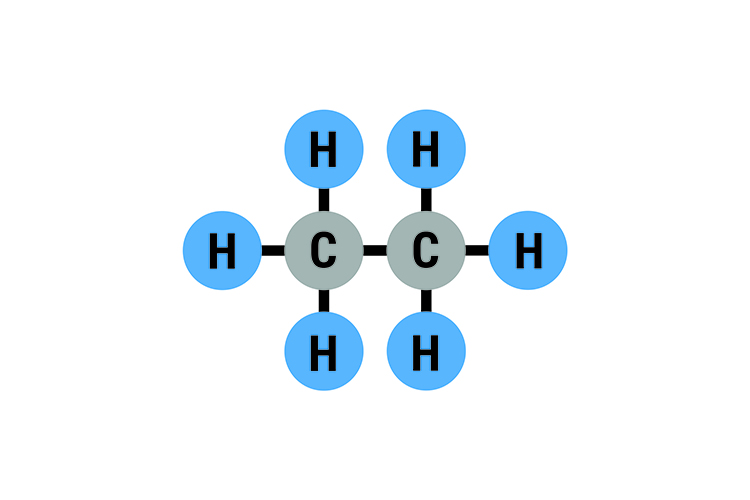
Ethane C2H6 – Boiling point -89°C (-127°F)
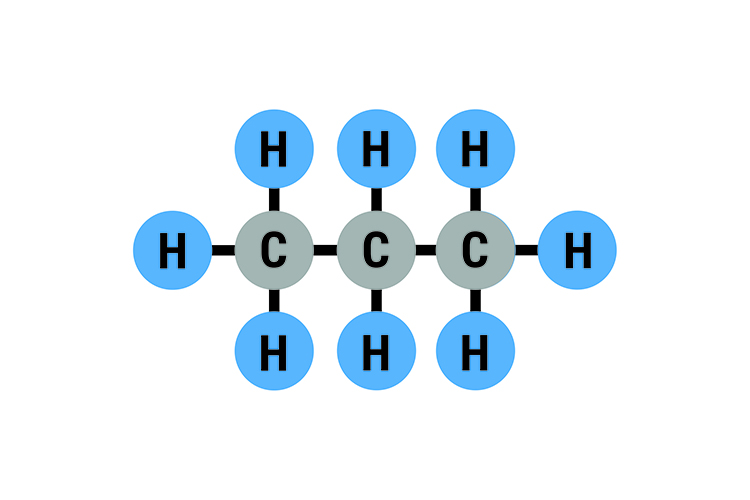
Propane C3H8: Boiling point -42°C (-44°F)
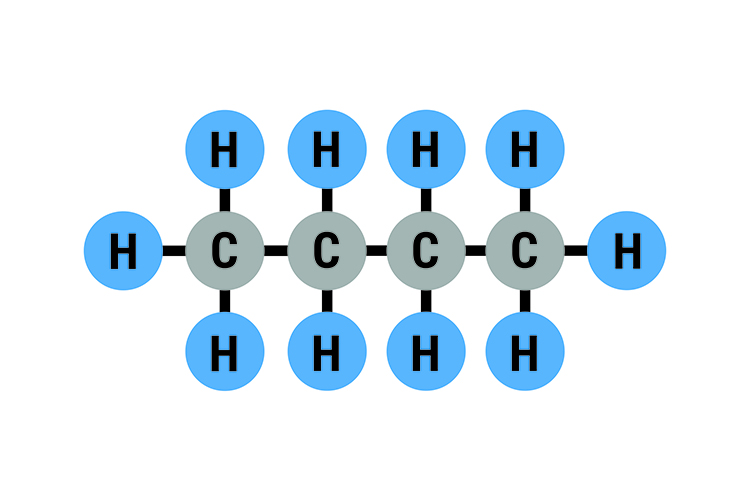
Normal butane C4H10: Boiling point -0.51°C (31°F)
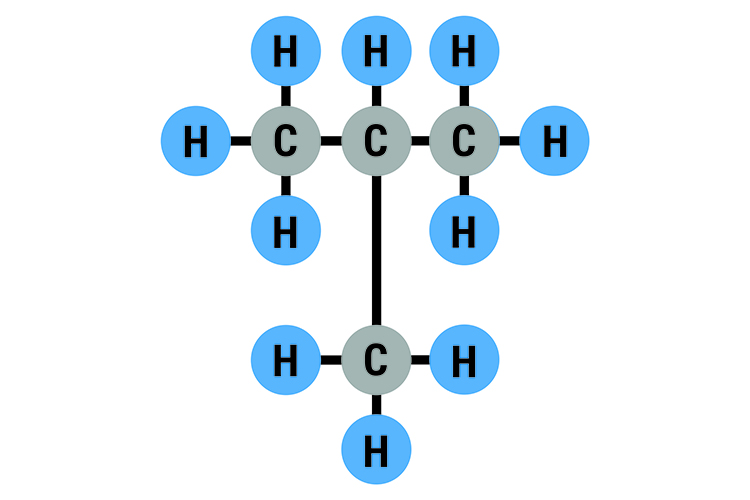
Isobutane C4H10: Boiling point -12°C (11°F)
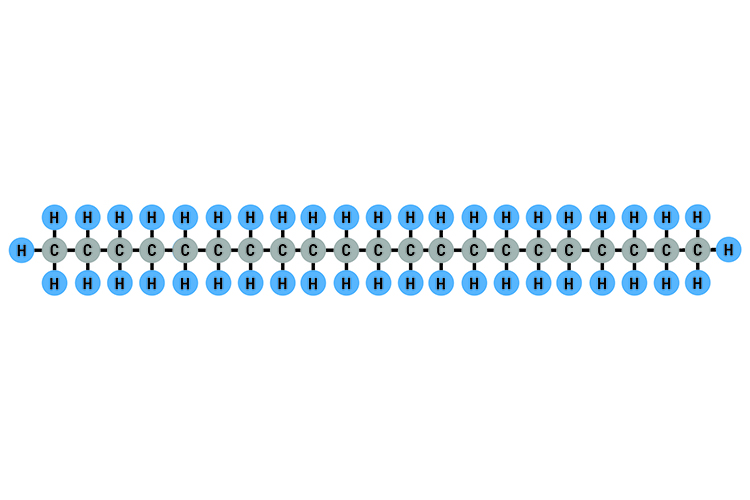
Heneicosane C21H44: Boiling point 100°C (212°F)