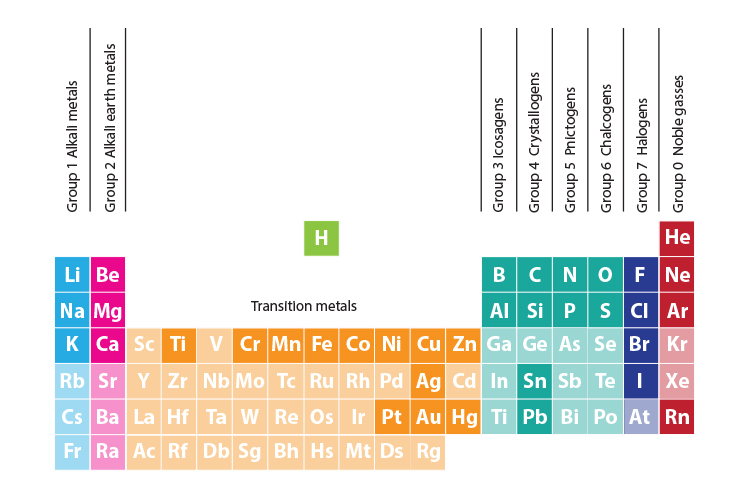
Groups of the periodic table
All of the elements in the periodic table are divided up into groups. All of the elements in a group behave in the same way. They have some pretty weird names, though, which are very difficult to remember… unless you use the Mammoth Memory story technique, of course!
Note:
The groups most discussed in school Chemistry are:
Group 1 Alkali metals
Group 2 Alkali earth metals
Group 7 Halogens
Group 0 Noble gases
and the Transition metals
To remember the names of the groups, use the following story:
We were talking about groups, which is a coincidence – I was out for a walk with a group of friends just the other day.
1. Alkali metals
.jpg)
Never call this group just alkalis, it should always be referred to as the alkali metals. Alkalis on their own are something entirely different. See our Acids and Alkalis section.
2. Alkaline earth metals
.jpg)
Never call this group just the alkalines; these elements should always be referred to as the alkaline earth metals. The word alkaline refers to something entirely different. See our Acids and Alkalis section.
3. Transition metals
.jpg)
4. Icosagens
.jpg)
We drove safely out of the alley and a very expensive jewellery store caught my eye – it was selling high cost gems. (Icosagens)
5. Crystallogens
.jpg)
6. Pnictogens
.jpg)
7. Chalcogens
.jpg)
8. Halogens
.jpg)
9. Noble gases
.jpg)
The angel presented the thief she had caught to the mayor. His clothes made him look very noble. (Noble gases)
Recap
So, to remind you, we…
- Had to walk down alley with alkies lying down. (Alkali)
- Saw alcohol in lines in the earth (Alkaline earth)
- Took a ride in a transit van (Transition)
- Stopped at a store selling high-cost gems (icosagens)
- Saw a necklace made of crystal… (crystallogens)
- …which got nicked (pnictogens)
- The storekeeper added things up on his calculator (chalcogens)
- An angel snared the thief with her halo (halogens)
- She took him to the mayor, who looked noble (noble gases)




