The history of the periodic table
In the 19th century a British chemist called Newlands (News lands) created a table of all the known chemical elements.
.jpg)
Newlands decided to arrange elephants (elements) on his table in order of their mass, but he left no gaps between each one for new elephants that had not yet been discovered.
.jpg)
As a result, Newlands’ table grouped elephants (elements) with different houses (properties) together.
Because there weren’t any gaps and elephants (elements) with different houses (properties) had been put next to each other, when the idea was presented, it was rejected by other scientists and the table was broken.
By mending and leaving (Mendeleev) gaps in the table, a Russian chemist called Mendeleev was able to keep elephants (elements) with similar houses (properties) together on the table.
The alterations Mendeleev had made to the table meant that it was met with approval by other scientists.
The history of the periodic table in more detail
John Newlands arrange the known elements in order of their increasing atomic mass.
Newlands observed that when the elements were ordered this way, every eighth element exhibited similar chemical properties, much like the notes in a musical scale. For example, sodium (Na) is the eighth element after lithium (Li) and both share similar reactive properties.
However, this pattern only worked well for the lighter elements, up to calcium, and his arrangement was not widely accepted at the time due to its limitations, such as placing elements with dissimilar properties in the same group and not leaving gaps for undiscovered elements.
The following is Newland's Octaves chart in 1865:
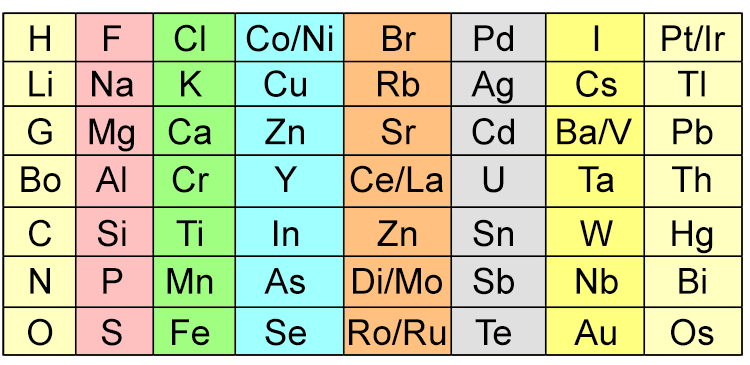
Note: Symbol names for elements have since changed.
The work Newland created paved the way for Dimitri Mendeleev who also decided to put the elements into atomic mass order. The main difference is that Mendeleev left gaps for undiscovered atoms and also grouped elements with similar chemical properties.
The following is Mendeleev's chart created in 1869:
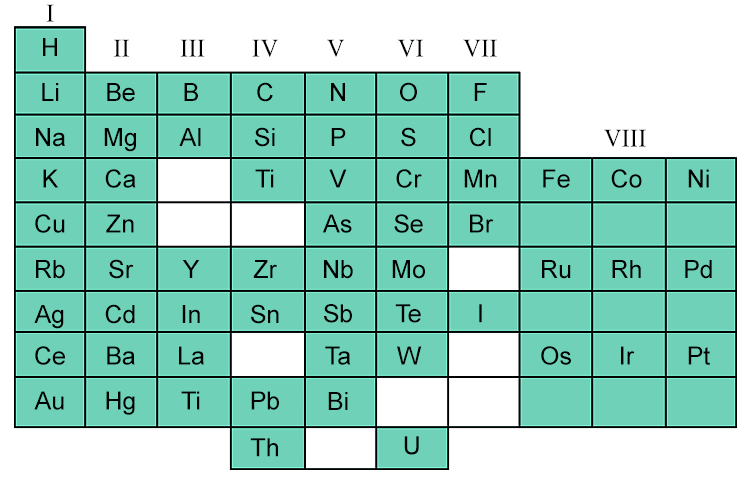
This became the foundation tool in chemistry. It changed in the 20th century because there were some slight inconsistencies. The transition from Mendeleev's chart to the modern periodic table involved major developments: the reordering by the atomic number (the number of protons), the discovery of noble gasses, and the addition of the lanthanide and the actinide series located below the main body of the periodic table.




