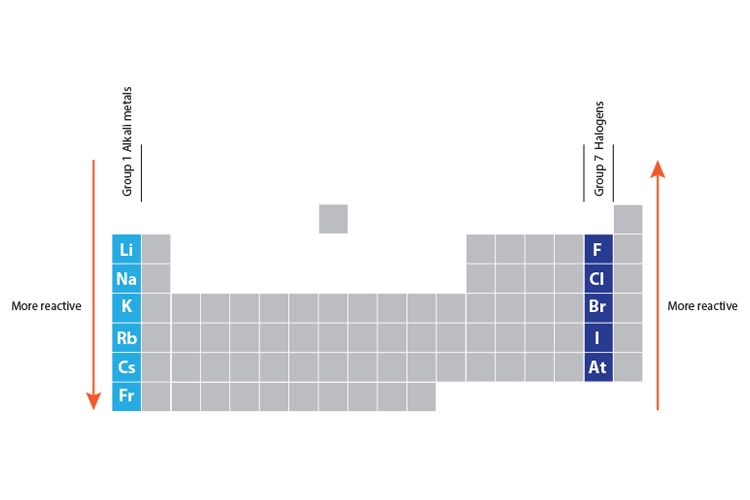
Reactivity of halogens and alkali metals
As you go down group 1 (the alkali metals) in the periodic table, the elements get more reactive.
As you go up group 7 (the halogens), again, the elements become more reactive.
Why do alkali metals get more reactive going down group 1?
Alkali metals from lithium to potassium get more reactive because the force of attraction between the nucleus (core) and the outer electron gets weaker as you go down group 1 elements.
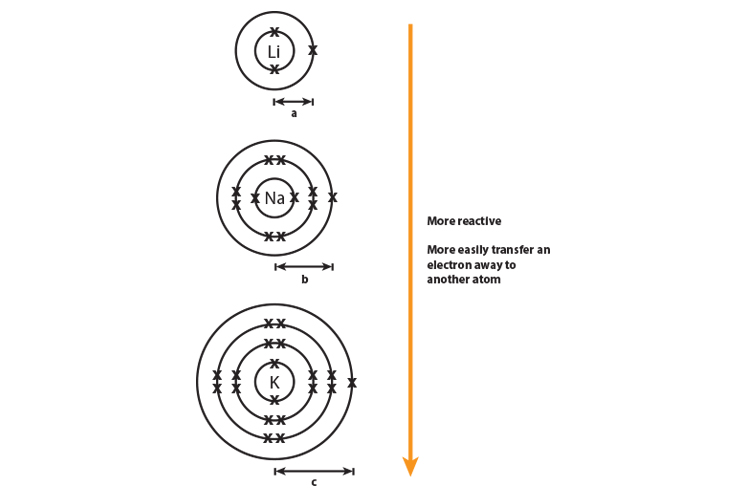
The distance "c" is greater than "a" and the force of attraction between the nucleus and the outer shell (rings) diminishes with distance.
The more electron shells (rings) between the nucleus and outer electron also creates shielding and again this weakens the nuclear attraction.
The outer electron is more easily transferred to say an oxygen atom, which needs electrons to complete its full outer shell.
Why do halogens get more reactive going upwards in group 7?
Halogens from bromide to fluorine get more reactive because the force of attraction between the nucleus (core) and the outer electron get stronger as you go up group 7 elements.
.4f413d0.jpg)
The distance "a" is less than "c" and the force of attraction between the nucleus and the outer shell increases with shorter distances.
The fewer electron shells (rings) between the nucleus and the outer shell (ring) also has less shielding effect and again this increases the electron attraction.
The outer shell will more easily attract another electron, which needs an electron to complete its full outer shell, when there is more attractive force.
A useful mnemonic picture to help you recall that:
As you go down group 1 (the alkali metals) in the periodic table, the elements get more reactive.
As you go up group 7 (the halogens), again the elements get more reactive.
Is as follows:
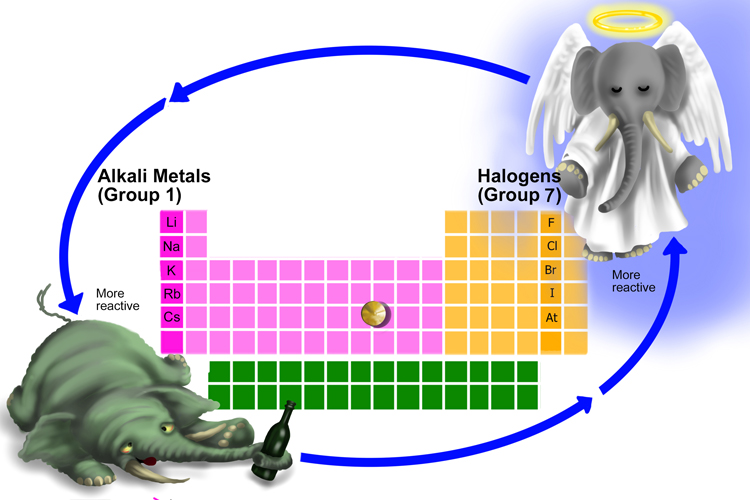
To remember how the reactivity of the alkali metals and halogens increases or decreases, put a pin in the middle of the periodic table and spin it anti-clockwise.
Drunks fall down, angels rise.
Summary
Group 1 metals become more reactive as you move down the periodic table because the outer single electron becomes easier to lose. This is due to a decrease in the attractive force between the nucleus and the outer electron as the outer electron is further from the nucleus and experiences more shielding from inner electrons.
Group 7 halogens become more reactive as you move up the periodic table because the single electron required to fill the outer shell has a stronger attraction for electrons. This is due to an increase in the attractive force between the nucleus and the outer electron it needs as it is closer to the nucleus and there is less shielding from inner electrons.




