Properties of the Noble Gases
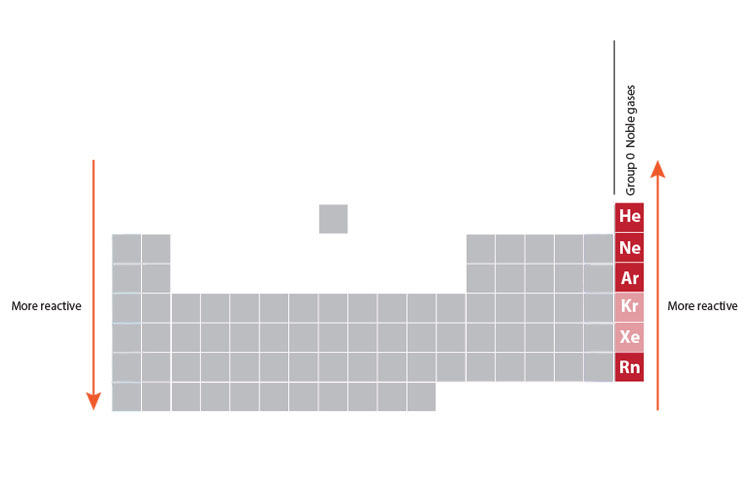
The elements in group 0 (Yes, group 0 - there are 8 groups but they are numbered 1 to 7 and then 0, not 1 to 8) of the periodic table are referred to as the noble gases, as you found out in the story about the names of the groups. But what do you need to know about these elements and the way they behave? Well, they’re colourless and non-reactive, and the elements near the bottom of the Noble Gases column are denser than the ones at the top. How do you remember that? Read on!
The Noble Gases

When I was in the UK, I took a tour of the Houses of Parliament with the mayor, who as we know is very noble. (Noble gases)
Colourless

Sadly, the politicians weren’t doing anything and the inside of the building was really boring and colourless.
Non-reactive
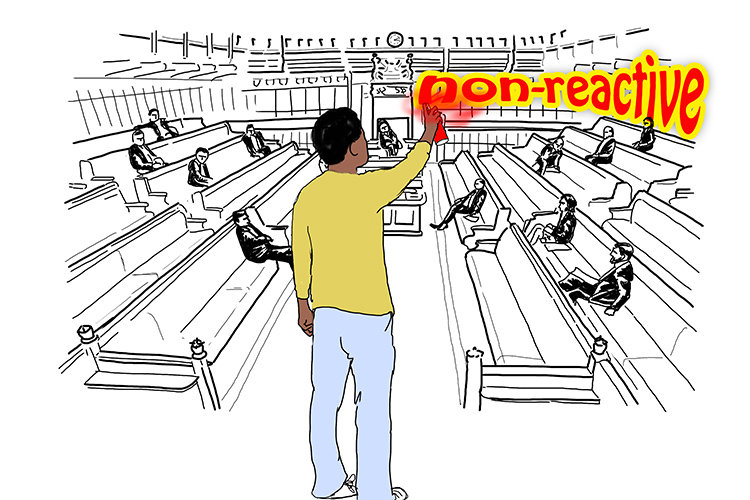
I wanted to try to wake them up a bit, so I tried doing something rebellious. I started spraying graffiti on the walls, but they still didn’t react! (Non-reactive)
Denser as you go down

While I was looking for a reaction, I noticed that the politicians on the lower benches didn’t really know what was going on. They seemed very dense. (Denser as you go down).
Summary
so ...
- The Noble Gases (Mr. Mayor) are
- Colourless (the inside of the boring, colourless building)
- Non-reactive (no reaction to the graffiti)
- Denser as you go down (the dense politicians are on the lower benches)




