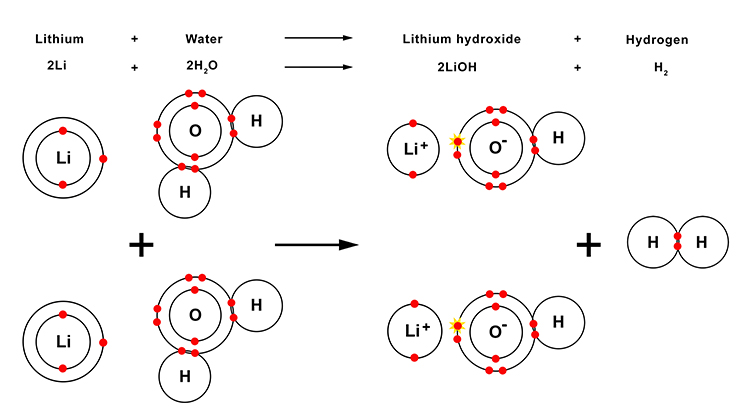
The reaction between lithium and water
When lithium reacts with water, lithium hydroxide and hydrogen gas are produced. Lithium hydroxide is an alkali.
| Lithium | + | Water | → | Lithium hydroxide | + | Hydrogen |
| 2Li | + | 2H2O | → | 2LiOH | + | H2 |
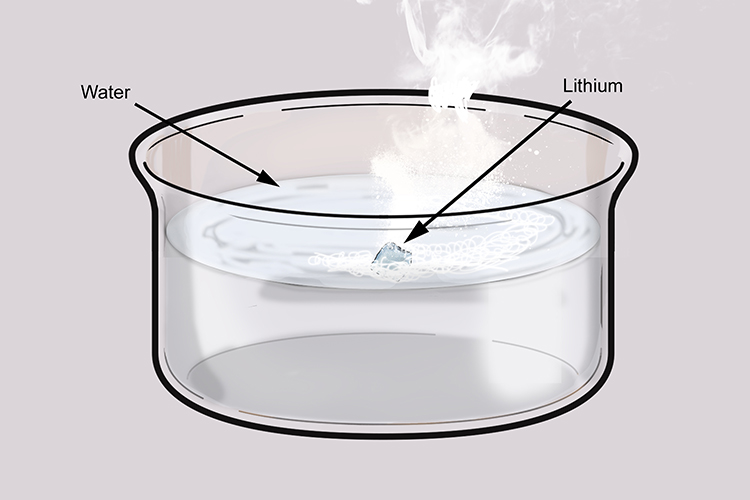
When lithium reacts with water it does not produce heat quickly enough to ignite the hydrogen given off or melt the lithium, which has a higher melting point than potassium and sodium.
The reaction between lithium and water in more detail
Note: You will not need to know this information but it may help to provide you with a better understanding of what happens in this reaction.