The reaction between sodium and water
When sodium reacts with water, sodium hydroxide and hydrogen gas are produced. Sodium hydroxide is an alkali.
| Sodium | + | Water | → | Sodium hydroxide | + | Hydrogen |
| 2Na | + | 2H2O | → | 2NaOH | + | H2 |
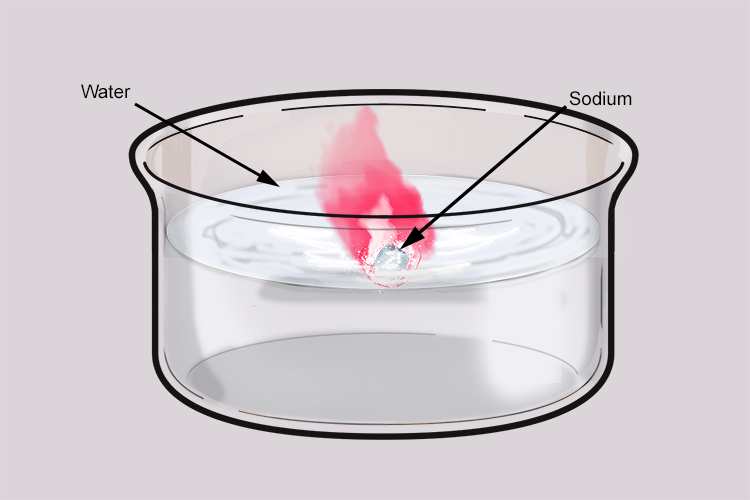
Sodium and water also react vigorously. Sodium has a low melting point so the heat given off in the reaction is enough to melt the sodium and cause it to form a ball. As hydrogen is produced, it pushes the ball of sodium around on the surface of the water. Sometimes, the hydrogen can ignite and produce an orange flame due to the sodium impurities it contains.
The reaction between sodium and water in more detail
Note: You will not need to know this information but it may help to provide you with a better understanding of what happens during this reaction.
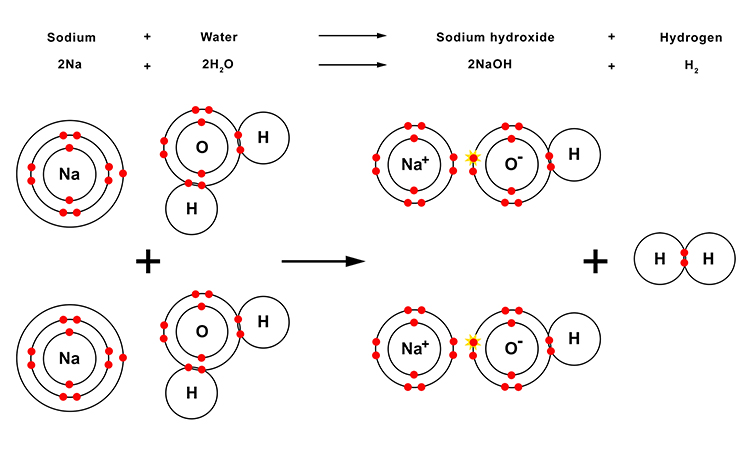
As when other metals react with water, the oxygen atom (O) in the resulting hydroxide molecule (NaOH) has both an ionic bond with the metal (sodium (Na)) atom and a covalent bond with the hydrogen (H) atom.




