The reaction between iron and steam
Of the metals that will react with steam, iron is the least reactive.
| Iron | + | Steam | ↔ | Iron(III) oxide | + | Hydrogen |
| 2Fe | + | 3H2O | ↔ | Fe2O3 | + | 3H2 |
Unlike other reactions between metals and steam, the reaction between iron and steam is reversible (shown by the arrows going in both directions).
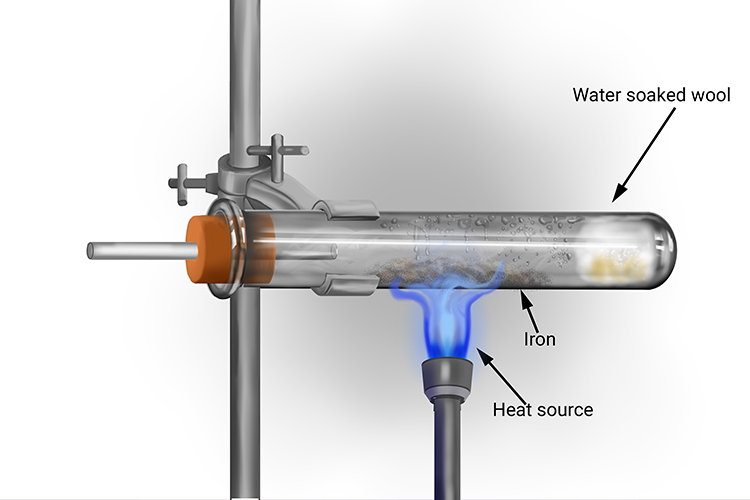
The reaction between iron and steam is much slower than the reactions between steam and metals higher in the reactivity series.
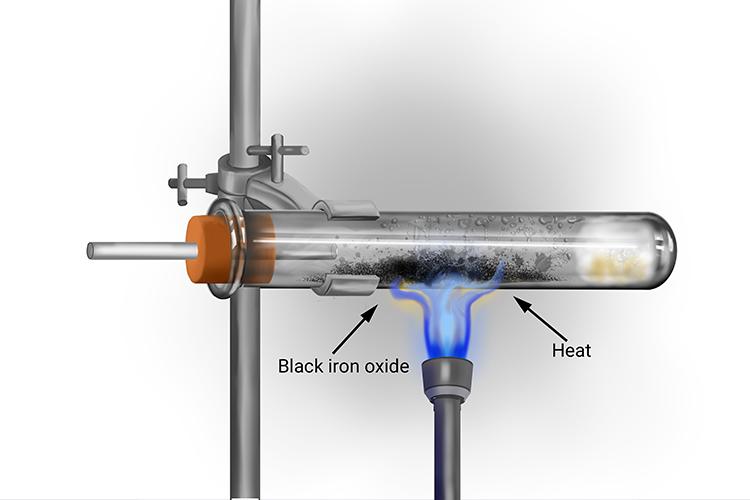
Heat often has to be applied to the iron in the test tube to initiate the reaction with steam and speed it up. Even when this is done, the iron does not glow and burn like magnesium does. Instead, the iron turns black as the iron oxide is formed.
The reaction between iron and steam in more detail
Note: You will not need to know this information but it may help to provide you with a better understanding of what happens during this reaction.
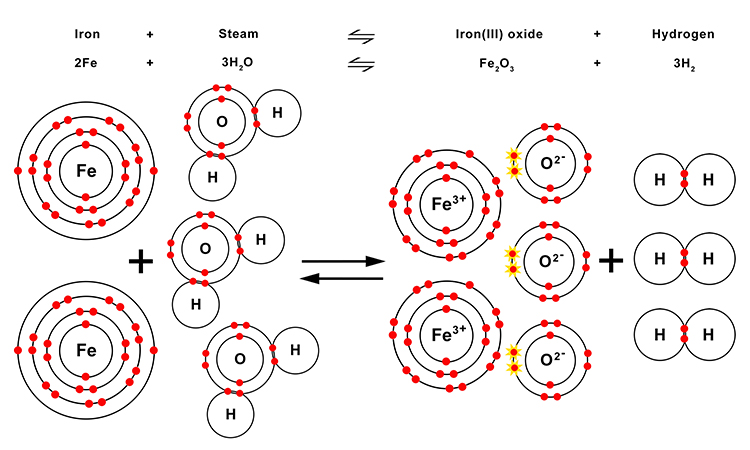
When the iron atoms react with the steam molecules they lose the two electrons from their outer (valence) shell and one from the next shell to form the iron (III) ion of the iron oxide.




