Isomers
Isomers are chemicals that have the same molecular formula but a different arrangement of atoms.

Isomers have the same chemical formula, but their atoms are arranged differently.
Because the arrangement of the atoms within the molecule is different, the strength of the bonds between the molecules (the intermolecular forces) can vary. This results in the isomers having different physical properties. For instance, isomers held together by stronger intermolecular forces have higher melting and boiling points than those held together by weaker bonds.
Isomers occur within homogenous series such as alkanes, alkenes and alcohols.
With alkanes, when they contain up to three carbon atoms, there is only one possible structure for each molecular formula. However, when there are four or more carbon atoms in an alkane, it is possible for it to have different structures.
For example, there are two isomers of butane C4H10: butane and isobutane.
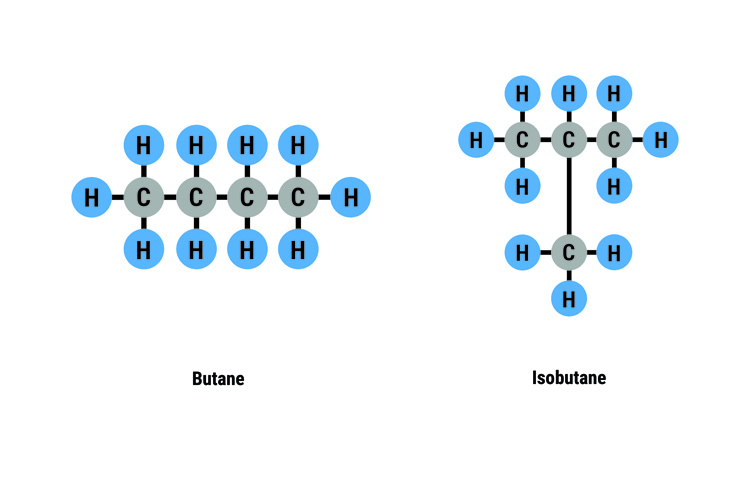
In each structure, every carbon atom is joined to 4 other atoms and every hydrogen atom is joined to one other atom.
The more carbon atoms an alkane has, the more common isomerism becomes. Whilst there are 5 isomers of hexane C6H14, there are 75 isomers of decane C10H22 and 159 isomers of undecane C11H24!




