The reaction between aluminium and steam
Like the reaction between magnesium and water, it is possible to react aluminium with steam. However, the aluminium oxide produced forms a barrier on the surface of the aluminium which prevents further reaction.
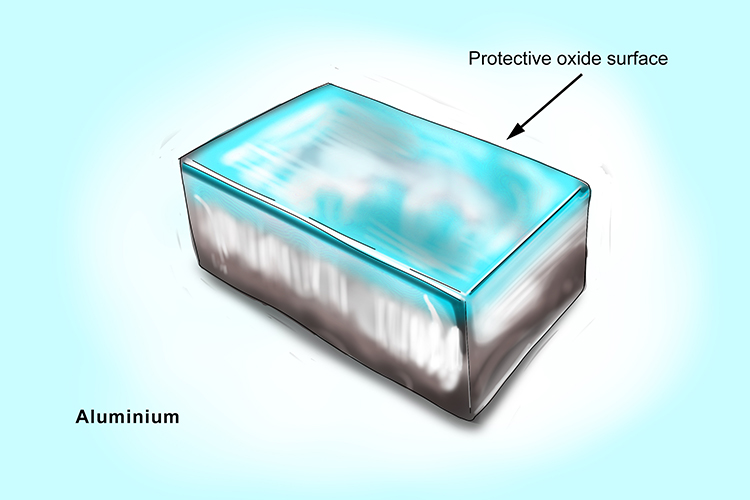
When aluminium reacts with steam, the products are aluminium oxide and hydrogen gas.
| Aluminium | + | Steam | → | Aluminium oxide | + | Hydrogen |
| 2Al | + | 3H2O | → | Al2O3 | + | 3H2 |
The reaction between aluminium and steam in more detail
Note: You will not need to know this information but it may help to provide you with a better understanding of what happens during this reaction.
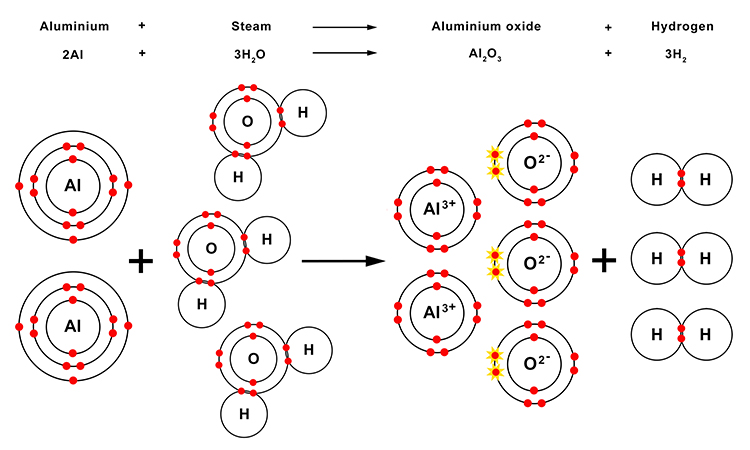
As with the magnesium oxide in the earlier reaction, the aluminium and oxygen that make up the aluminium oxide are held together by ionic bonds.




