The reaction between magnesium and steam
When magnesium reacts with steam, magnesium oxide and hydrogen gas are produced.
| Magnesium | + | Steam | → | Magnesium oxide | + | Hydrogen |
| Mg | + | H2O | → | MgO | + | H2 |
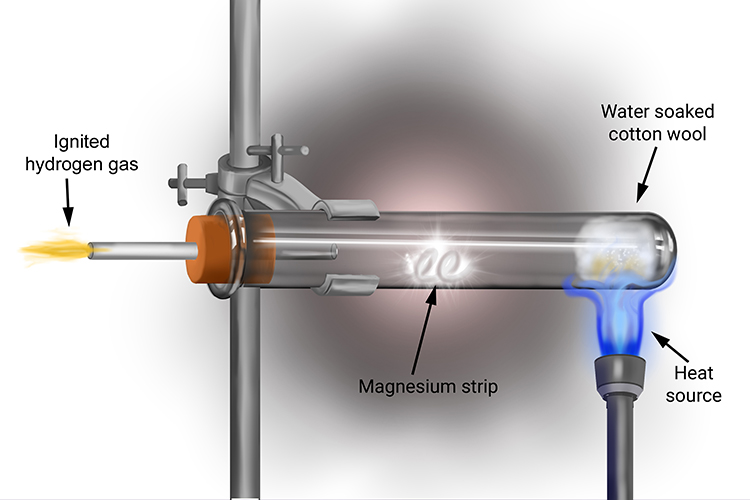
When the cotton wool is heated, the water turns to steam; this reacts with the magnesium strip in the tube to produce hydrogen gas. If a lit splint is placed at the end of the tube, the hydrogen gas produced will ignite.
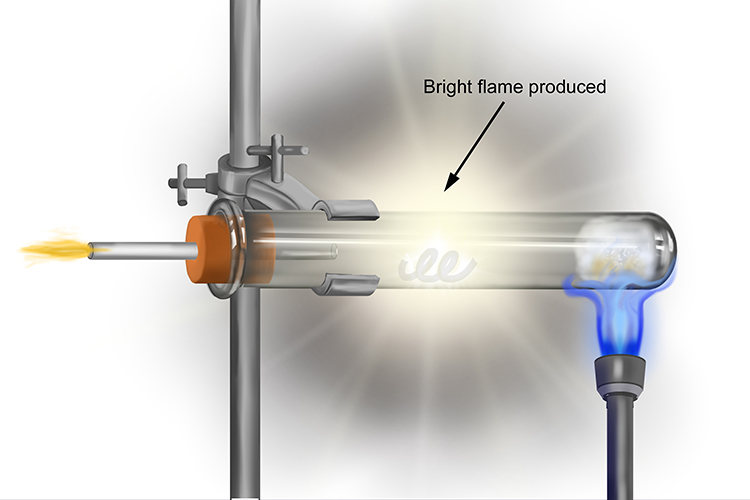
This reaction produces a bright flame from the magnesium strip as it burns in the presence of the steam. It isn’t long before all the magnesium is burnt.
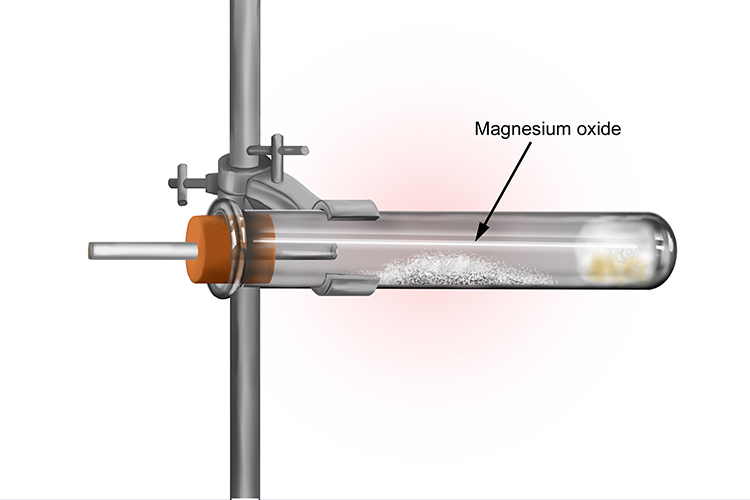
When the reaction is over there is a white ash left in the tube; this is the magnesium oxide.
The reaction between magnesium and steam in more detail
Note: You will not need to know this information but it may help to provide you with a better understanding of what takes place during this reaction.
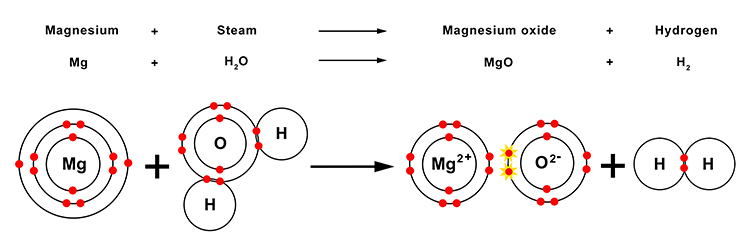
The magnesium and oxygen that make up the magnesium oxide are bonded together with an ionic bond, while the hydrogen atoms form a covalent bond.




