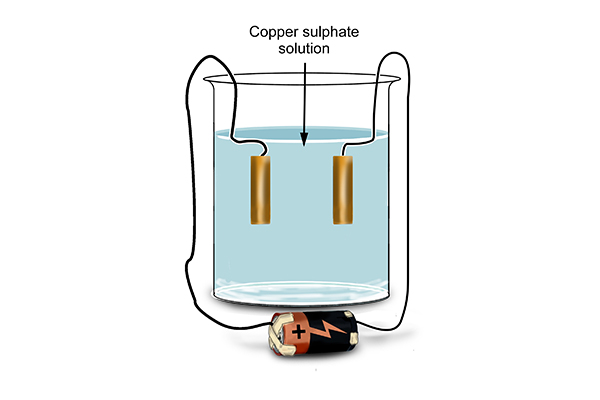
Example 2 - Electrorefining of copper
Instead of using inert electrodes, chemists tried placing pure copper electrodes in copper sulphate solution.
They found that the copper on the cathode became bigger and the copper on the anode became smaller.
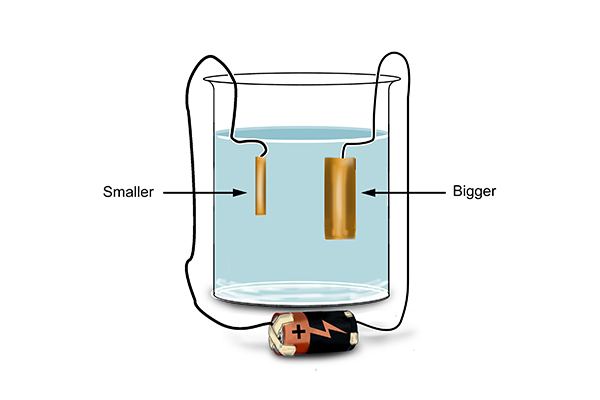
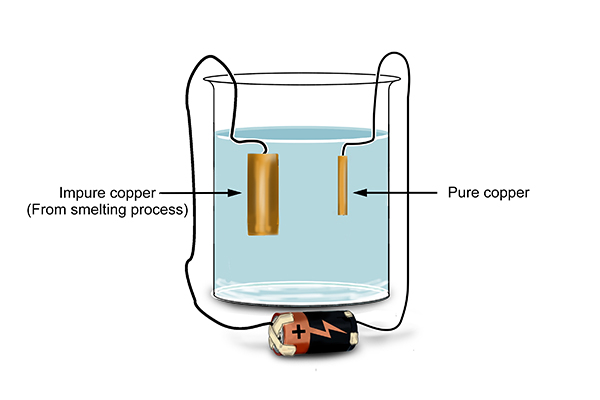
So the chemists then tried putting impure copper at the anode (copper from smelting).
NOTE: Pure copper is a better electrical conductor than impure copper. The chemists could make a lot of money if they could gather pure copper. Copper can be extracted from its ore using a smelting furnace, where copper is displaced by carbon (see reactivity series), but it is not pure – it has some impurities in it.
The chemists got pure copper.
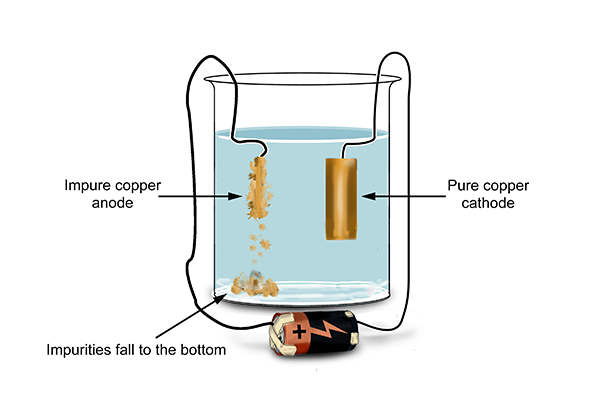
Wow, did the chemists get rich!