Rule 2: Electrolysis and halogens
If the solution on which electrolysis is being performed contains a halogen (such as chlorine and bromine), then the halogen will always form at the anode. If there is no halogen in the solution, then oxygen is formed at the anode.
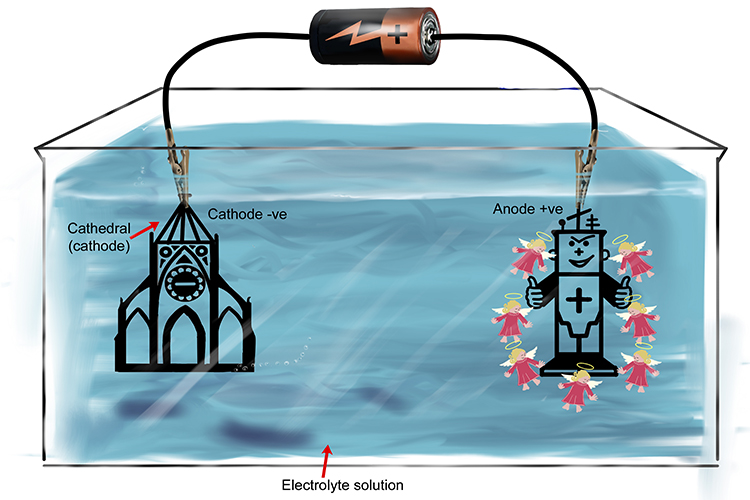
When a solution contains halogens (angels with haloes), they are attracted to the annoyed android (anode) as they want to soothe him and stop him being so annoyed.
Halogen > oxygen > all other negatively-charged ions




