Oxidation and reduction
Because we now know that:
- Cathodes give out electrons and gives an electron to a nearby element. That element gains an electron.
- Anodes take electrons from a nearby element. That element loses an electron.
And because:
- An element that has a loss of an electron is oxidation
- An element that has a gain of an electron is reduction
(Remember OIL RIG – Oxidation is loss, reduction is gain)
Reduction takes place at the cathode

Therefore oxidation takes place at the anode.
Another great mnemonic to remember that reduction takes place at the cathode and oxidation takes place at the anode is:

And a picture mnemonic of this would be:
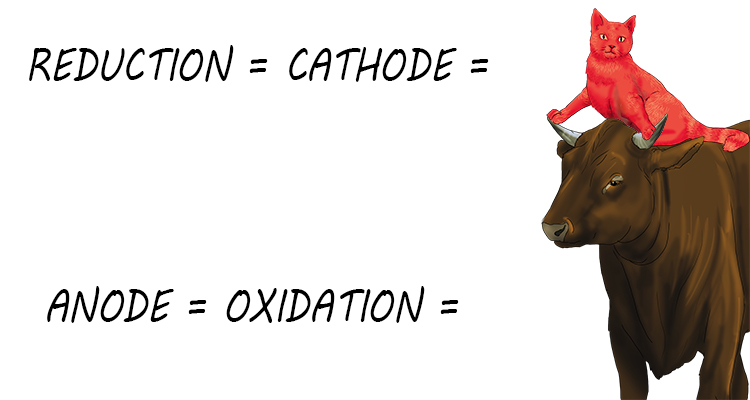
Reduction is cathode is RED CAT.
Anode is oxidation is AN OX.




