Example 3 - Electrolysis of water
The chemists even tried electrolysis on water.
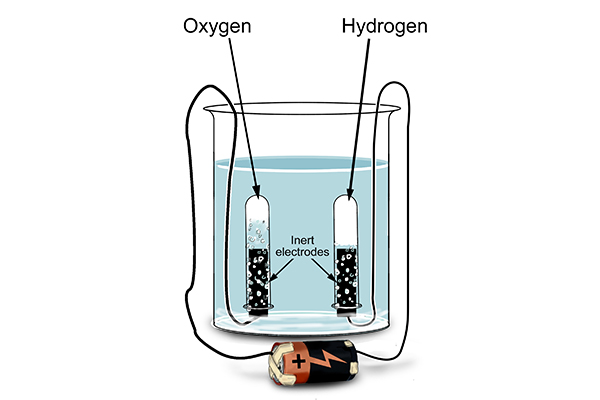
When they tested the tubes after performing electrolysis on water …
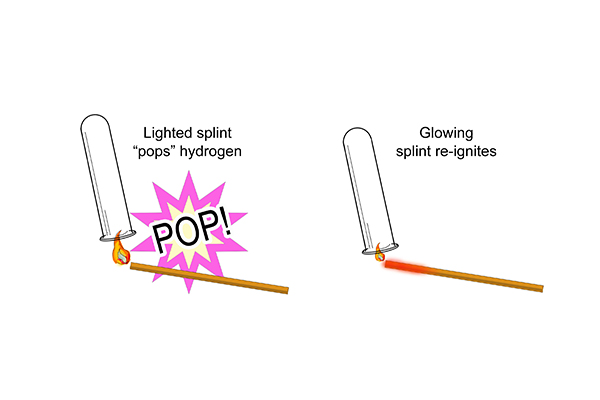
What was really interesting was that when they looked closely at the test tubes, twice as much volume of hydrogen had been created as there was oxygen. This is because there are two hydrogen atoms for every one oxygen atom in a molecule of water – as indicated by the formula, H2O.
See the examples at the end of this section for more detailed information, but remember …
- A mole of oxygen (O2) = 24 litres volume
- A mole of hydrogen (H2) = 24 litres volume




