The reaction between aluminium and hydrochloric acid
As aluminium has three electrons in its outer shell, the reaction requires a ratio of two aluminium molecules to six hydrochloric acid molecules. This is because each chlorine atom in the hydrochloric acid acquires an electron from the aluminium and loses a hydrogen atom. As hydrogen does not like to exist as a single atom, six molecules of hydrochloric acid are required to give three molecules of the diatomic hydrogen molecule, so two molecules of aluminium are required to keep things balanced.
| Aluminium Hydrochloric acid | → | Aluminium chloride | + | Hydrogen | ||
| 2Al + 6HCl | → | 2AlCl3 | + | 3H2 |
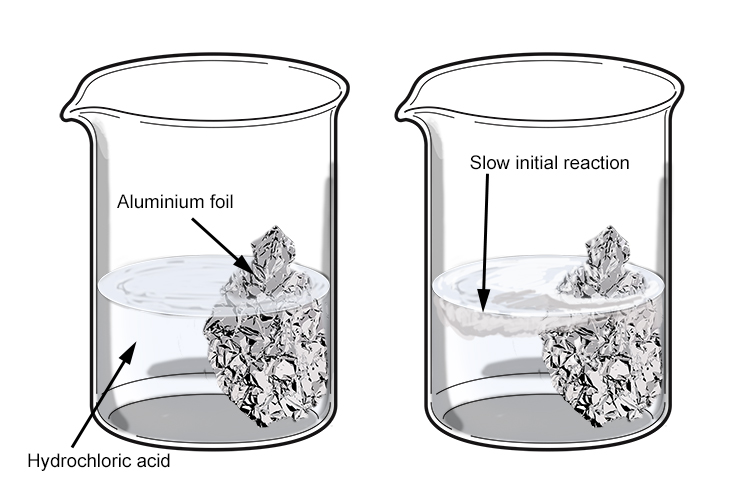
When aluminium is placed in an acid it may initially appear not to react. This is because a layer of aluminium oxide forms on the surface of the aluminium due to prior reaction with the air and acts as a protective barrier. The acid must remove this layer before it is able to react with the aluminium underneath.
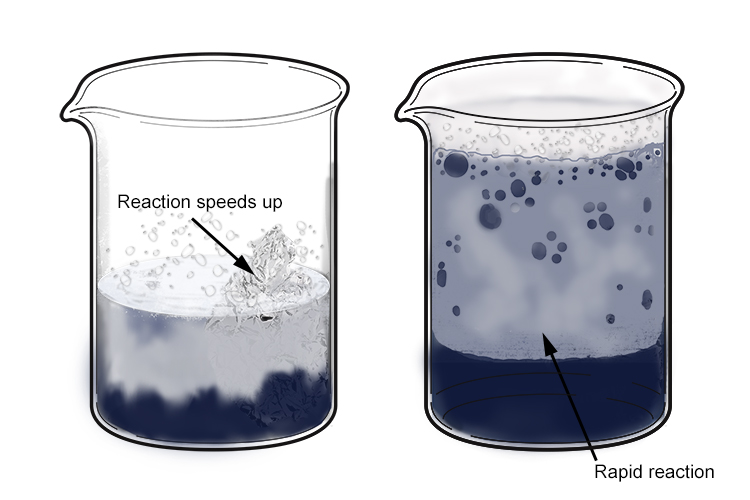
Once the acid has removed the oxide layer from the aluminium, the reaction speeds up significantly and produces a lot of hydrogen bubbles. The hydrochloric acid quickly turns a dull grey colour as aluminium chloride is formed.