The reaction between potassium and hydrochloric acid
When potassium reacts with hydrochloric acid, the salt produced is potassium chloride. The reaction is very quick due to potassium’s position in the reactivity series, although the speed of the reaction can also be affected by the strength of the acid.
| Potassium | + | hydrochloric acid | → | Potassium chloride | + | Hydrogen |
| 2K | + | 2HCl | → | 2KCl | + | H2 |
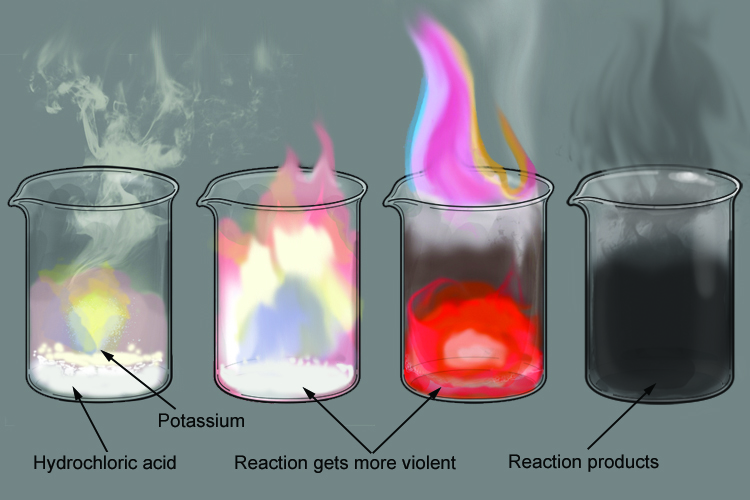
The reaction between potassium and hydrochloric acid is over very quickly. The potassium immediately ignites on contact with the acid, producing a bright lilac flame that quickly grows until the potassium burns up.




