The reaction between calcium and hydrochloric acid
Being a group 2 element, calcium is less reactive than the metals in group 1 of the periodic table.
The salt produced when calcium reacts with hydrochloric acid is calcium chloride.
| Calcium | + | Hydrochloic acid | → | Calcium chloride | + | Hydrogen |
| Ca | + | 2HCl | → | CaCl2 | + | H2 |
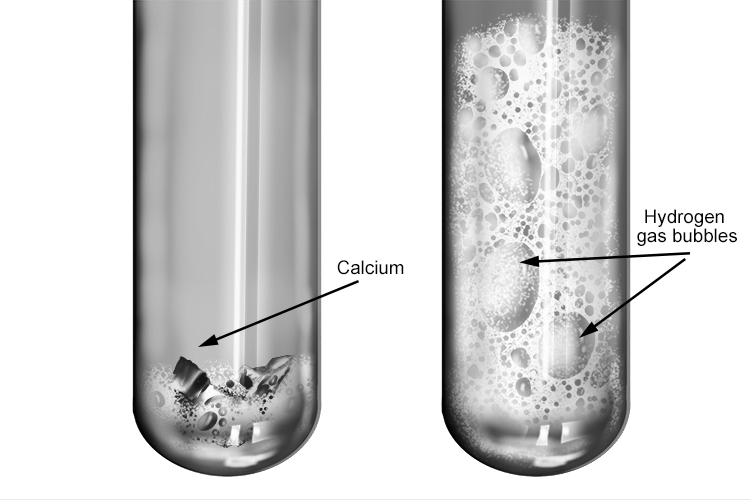
The reaction between calcium and hydrochloric acid is slower and less violent than when group 1 metals react with hydrochloric acid. Less heat is produced, so the hydrogen gas does not ignite.




