The reaction between lithium and sulphuric acid
The salt produced when lithium reacts with sulphuric acid is lithium sulphate.
| Lithium | + | Sulphuric acid | → | Lithium sulphate | + | Hydrogen |
| 2Li | + | H2SO4 | → | Li2 SO4 | + | H2 |
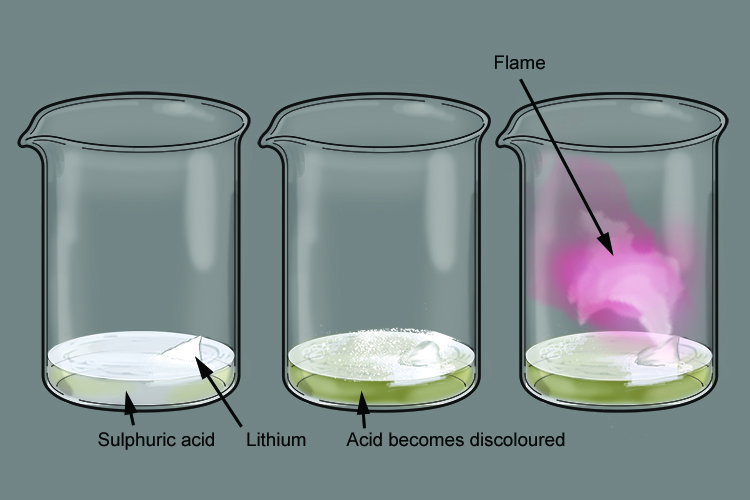
The lithium immediately starts to fizz as it gives off hydrogen gas on contact with the sulphuric acid. The sulphuric acid turns a yellow colour as it is converted to lithium sulphate.
The heat produced in the reaction ignites the hydrogen gas given off, producing a reddish flame.

As the reaction intensifies, so does the flame, until all the lithium is used up.




