Examples of giant covalent structures
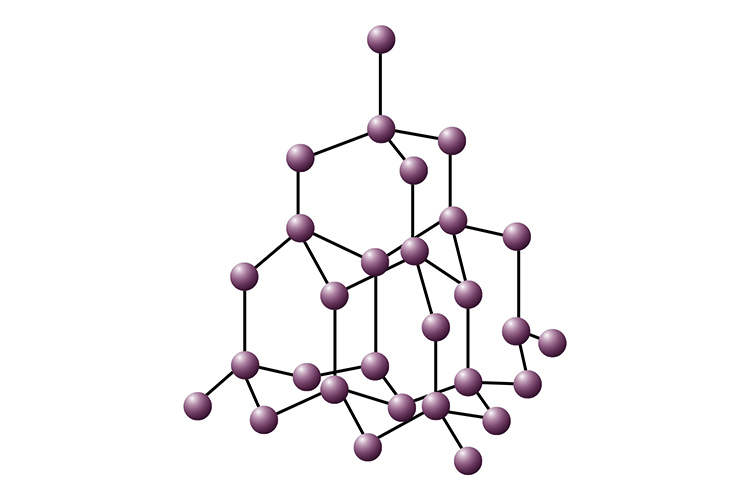
Diamond
Diamond is made of only carbon atoms. Each carbon atom forms four covalent bonds to make a giant covalent structure.
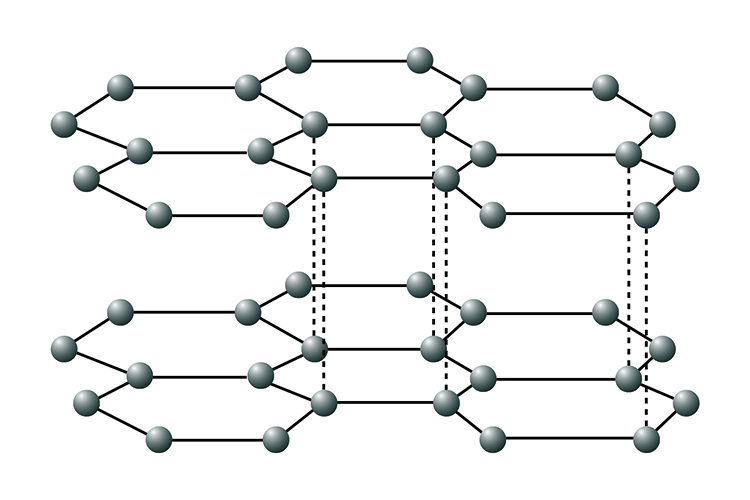
Graphite
Graphite also consists of just carbon atoms. Each atom forms three covalent bonds. This creates layers that can slide over one another. The layers are held together by free electrons which enable graphite to conduct heat and electricity (it is the only non-metal that is able to do this).
Silicon dioxide (also known as silica, a major constituent of sand)
Within silicon dioxide, each silicon atom is bonded to four oxygen atoms and each oxygen atom is bonded to two silicon atoms.
![]()




