Examples of ionic substances
Sodium chloride NaCl (salt)
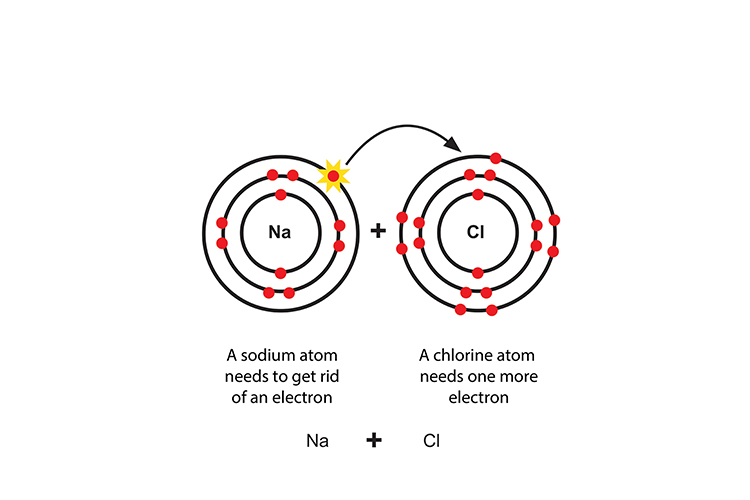
Sodium gives its extra electron to chlorine to form a sodium cation and a chloride anion.
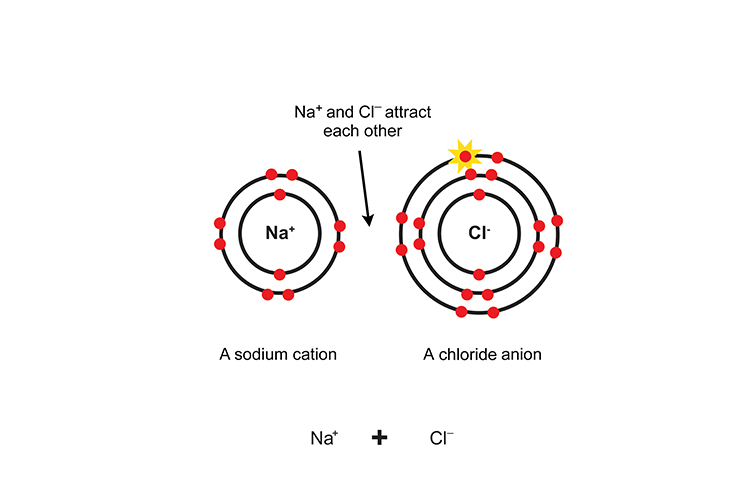
The two ions attract like magnets and are joined by an ionic bond.
Calcium chloride CaCl2
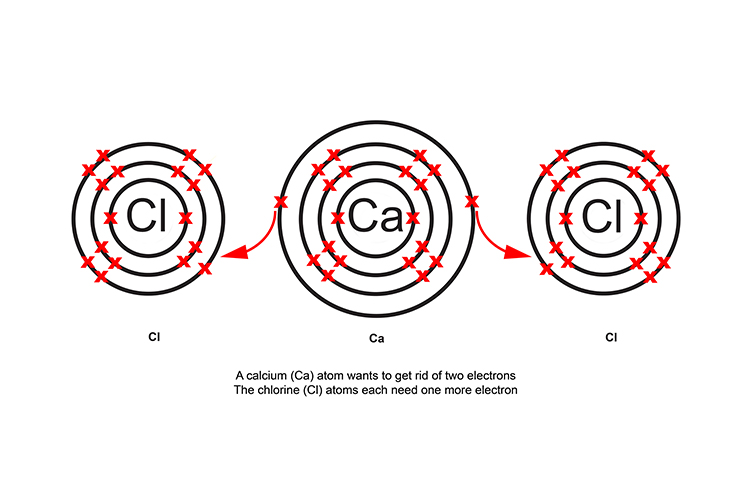
When calcium and chlorine react, the calcium atom transfers one electron to each of the two chlorine atoms.
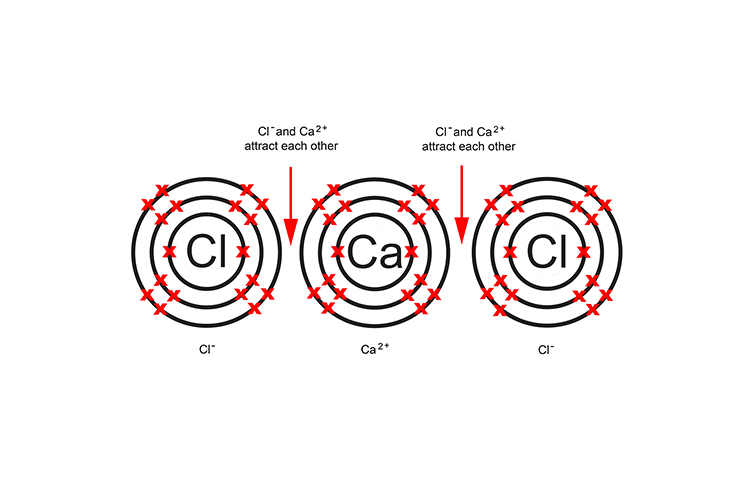
This results in one positive calcium ion and two negative chloride ions. The ions now attract like magnets, forming two ionic bonds.




