Ionic bonding (between metals and non-metals)
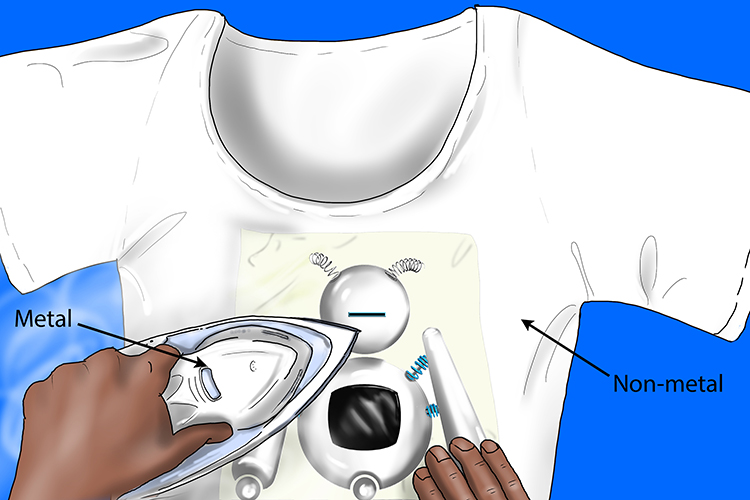
Ionic bonding is the transfer of electrons from a metal (iron) to a non-metal (t-shirt).






Ionic bonding is the transfer of electrons from a metal (iron) to a non-metal (t-shirt).